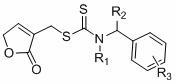
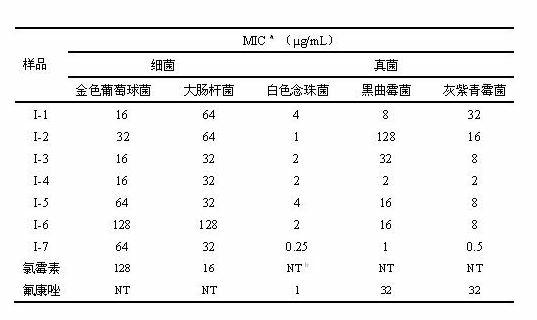
Butenolide structure-containing benzyl amino dithio formiate compounds, and preparation method and application thereof
A technology of benzylcarbamate and butenolactone, which is applied in the field of medicinal chemistry to reduce costs and increase antifungal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
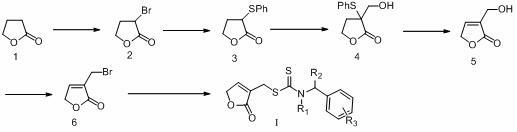
[0018] Compound 6 was prepared with reference to the following literature:
[0019] (a) B. Morgan, D. Dolphin, R. H. Jones, T. Jones, F. W. B. Einstein, J. Org. Chem. 52 (1987) 4628-4631. (b) H. S Yang, X. X. Qiao, Q. Cui, X. H. Xu, Chin. Chem. Lett. 20 (2009) 1023-1023.
Embodiment 1
[0020] Example 1, Synthesis of α-Bromo-γ-butyrolactone (Compound 2)
[0021] Under the condition of ice bath, slowly drop 6.4mL (125mmol) liquid bromine into the mixture of 10g (116mol) γ-butyrolactone and 13.4g (432mol) red phosphorus; mL (125mmol) liquid bromine. After the dropwise addition was completed, the reaction was maintained at 80°C for 3 h. Cool and blow in air continuously for 1 h to blow away excess liquid bromine and hydrogen bromide generated. Then the temperature was raised to 80°C, and 1.5 mL of distilled water was slowly added dropwise, and 20 mL of water was added after the reaction eased, and refluxed for 4 hours. After cooling, the layers were separated, and the aqueous layer was extracted three times with ether; the organic phases were combined and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure to obtain 19.6 g of crude compound 2.
Embodiment 2
[0022] Example 2, Synthesis of α-phenylene sulfide-γ-butyrolactone (compound 3)
[0023] Under the conditions of ice-salt bath and nitrogen protection, 5.1g (94 mmol) of sodium methoxide was added to 350mL of dry methanol, and then 12.6mL (122mmol) of thiophenol was added dropwise. After 15 min, a solution of 15.57 g (94 mmol) of compound 2 in 100 mL THF (tetrahydrofuran) was added and added dropwise slowly for 1 h. Remove the ice bath and stir at room temperature for 2 h. Evaporate the solvent under reduced pressure, add 200mL ethyl acetate, add diatomaceous earth and suction filter, combine the washed ethyl acetate, and then successively wash with saturated NaHCO 3 aqueous solution, NaOH aqueous solution and distilled water extraction. The ethyl acetate phase was collected and dried over anhydrous magnesium sulfate. After filtering and evaporating the solvent under reduced pressure, 18.67 g of the crude product of compound 3 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com