Sanguinarine alcoholate and chelerythrine alcoholate and preparation method and application thereof in animal acaricidal drugs
A technology of chelerythrine and alcoholate, which is applied in the direction of botanical equipment and methods, applications, acaricides, etc., can solve the problems of bad, recurrent and frequent repeated infections, etc., and achieve strong acaricidal activity and low residue production Drug resistance, the effect of not easy to produce drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
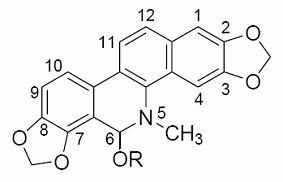
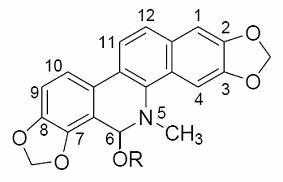
[0036] 1. The preparation method of sanguinarine alcoholate:
[0037] Add 1.00 mmol of sanguinarine and 50 mL of methanol, ethanol, propanol, or isopropanol into a 100 mL round-bottom flask, heat until the solids are completely dissolved; then add dropwise 0.5 mol / L of the corresponding alcohol sodium alkoxide, namely methanol Sodium, sodium ethoxide, sodium propoxide or sodium isopropoxide solution 4.0 mL, stirred at room temperature for 30 minutes, evaporated the solvent to dryness under reduced pressure; dissolved the residue with 50 mL chloroform, filtered off the insoluble matter, and evaporated the filtrate to remove the solvent under reduced pressure to dryness; the resulting residue was recrystallized with the corresponding alcohol, namely methanol, ethanol, propanol or isopropanol, to obtain white crystals or solids, namely sanguinarine alcoholate, with a yield of 85% to 95%.
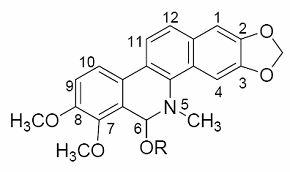
[0038] 2. The preparation method of chelerythrine alcoholate:
[0039] Add 1.00 mmol of ch...
Embodiment 1
[0043] 6-Methoxydihydrosanginarine, referred to as sanguinarine methanolate, the molecular structure is that R in the structure of sanguinarine alcoholate is replaced by a methyl group: light pink prism (methanol), mp 194-194.5℃. 13 C NMR (125 MHz, CD 3 OD) δ : 104.6(C-1), 148.1(C-2), 147.4(C-3), 100.6(C-4), 126.9(C-4a), 138.2(C-4b), 85.9(C-6), 108.8(C-6a), 145.3(C-7), 147.2(C-8), 113.2(C-9), 116.4(C-10), 125.8(C-10a), 122.8(C-10b), 120.1 (C-11), 123.7(C-12), 131.1(C-12a), 101.1(2,3-OCH 2 O), 101.7(7,8-OCH 2 O), 40.9(N-Me), 54.1(6-OMe). 1 H NMR (CDOD 3 , TMS) δ : 7.76(1H, d, J 8.6 Hz, H-11), 7.69(1H, s, H-4), 7.48(1H, d, J8.6 Hz, H-12), 7.20(1H, d, J 8.2 Hz, H-10), 7.12(1H, s, H-1), 6.93(1H, d, J 8.2 Hz, H-9), 6.11(2H, s, OCH 2 O), 6.04(2H, s, OCH 2 O), 5.37(1H, s, H-6), 3.46(3H, s, OCH 3 ), 2.79(3H, s, NCH 3 ); ESI-MS (positive mode) m / z : 364[M+H] + .
Embodiment 2
[0045] 6-Ethoxydihydrosanguinarine, referred to as sanguinarine alcoholate, the molecular structure is that R in the structure of sanguinarine alcoholate is replaced by ethyl: white prism (ethanol), mp 210-212℃. 13 C NMR (125 MHz, CD 3 OD) δ : 104.6(C-1), 148.0(C-2), 147.4(C-3), 100.7(C-4), 126.9(C-4a), 138.5(C-4b), 84.2(C-6), 108.7(C-6a), 145.2(C-7), 147.3(C-8), 113.4(C-9), 116.4(C-10), 125.8(C-10a), 122.9(C-10b), 120.3 (C-11), 123.6(C-12), 131.0(C-12a), 101.0(2,3-OCH 2 O), 101.7(7,8-OCH 2 O), 40.9(N-Me), 61.6(O C h 2 CH 3 ), 15.0 (OCH 2 C h 3 ). 1 H NMR (CDOD 3 , TMS) δ : 7.76(1H, d, J 8.6 Hz, H-11), 7.66(1H, s, H-4), 7.48(1H, d, J 8.6 Hz, H-12), 7.40(1H, d, J 8.2 Hz, H-10), 7.12(1H, s, H-1), 6.92(1H, d, J 8.2 Hz, H-9), 6.11(2H, s, OCH 2 O), 6.08(2H, s, OCH 2 O), 5.48(1H, s, H-6), 3.90-3.93(1H, m, OC H 2 CH 3 ), 3.65(1H, q, J 7.0 Hz, OC H 2 CH 3 ), 2.76(3H, s, NCH 3 ), 1.08(3H, t, J 7.0 Hz, OCH 2 C H 3 ); ESI-MS (positive mode) m / z : 378...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com