Alpha-galactosidase and encoding gene and application thereof
A technology of galactosidase and gene, applied in the field of α-galactosidase and its coding gene and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
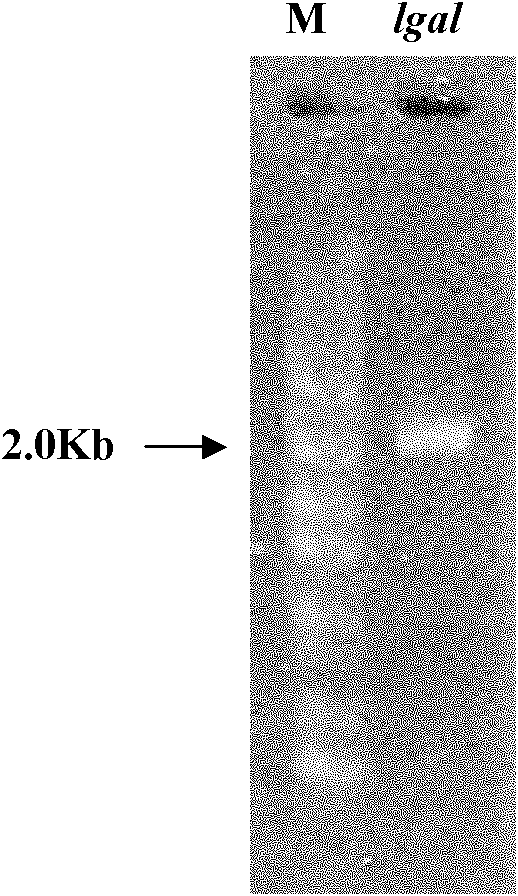
[0038] Example 1, the acquisition and identification of bacterial strain WD, the discovery of α-galactosidase (LGAL protein) and its coding gene (lgal gene)
[0039] 1. Acquisition and identification of strain WD
[0040] 1. Obtaining strain WD
[0041] Strain WD was independently screened from soybean planting soil in September 2008.
[0042] 2. Identification of strain WD
[0043] (1) Morphological characteristics of strain WD
[0044] Plate culture is black velvet or powdery, liquid medium culture, mycelium is black mass flocculent, growing rapidly.
[0045] (2) Molecular identification of strain WD
[0046] The genomic DNA of strain WD was extracted, and the 18sRNA sequence was obtained by PCR amplification (see sequence 4 in the sequence listing). The 18sRNA sequence shown in Sequence 4 was compared with the 18sRNA sequence of existing strains, and it was found that the similarity with the 18sRNA of Alternaria was the highest, reaching 99%. The strain WD was confirm...
Embodiment 2
[0141] Embodiment 2, the preparation of recombinant bacteria
[0142] 1. Preparation of recombinant expression vector
[0143] 1. Extract the RNA of the bacterial strain WD, reverse transcribe it into cDNA; use the cDNA as a template, and perform PCR amplification with a primer pair composed of LYF and LYR to obtain a PCR amplification product.
[0144] LYF: 5'-GTCGACATGTTTGGCATGAAGGGTGTTTG-3' (Add Sal I recognition sequence);
[0145] LYR: 5'-GCGGCCGCTCAGACCTTGTTCAACCAAAATCA-3' (Add Not I recognition sequence).
[0146] 2. The PCR amplification product was recovered, connected to PEASY-T3 Cloning Vector (purchased from Beijing Quanshijin Biotechnology Co., Ltd., catalog number CT301), and the positive clones were picked for sequencing. The sequencing results showed that the sequence 2 containing the sequence listing was obtained The recombinant plasmid of the indicated DNA was named as the recombinant plasmid pT3-lgal.
[0147] 3. Digest the recombinant plasmid pT3-lgal wi...
Embodiment 3
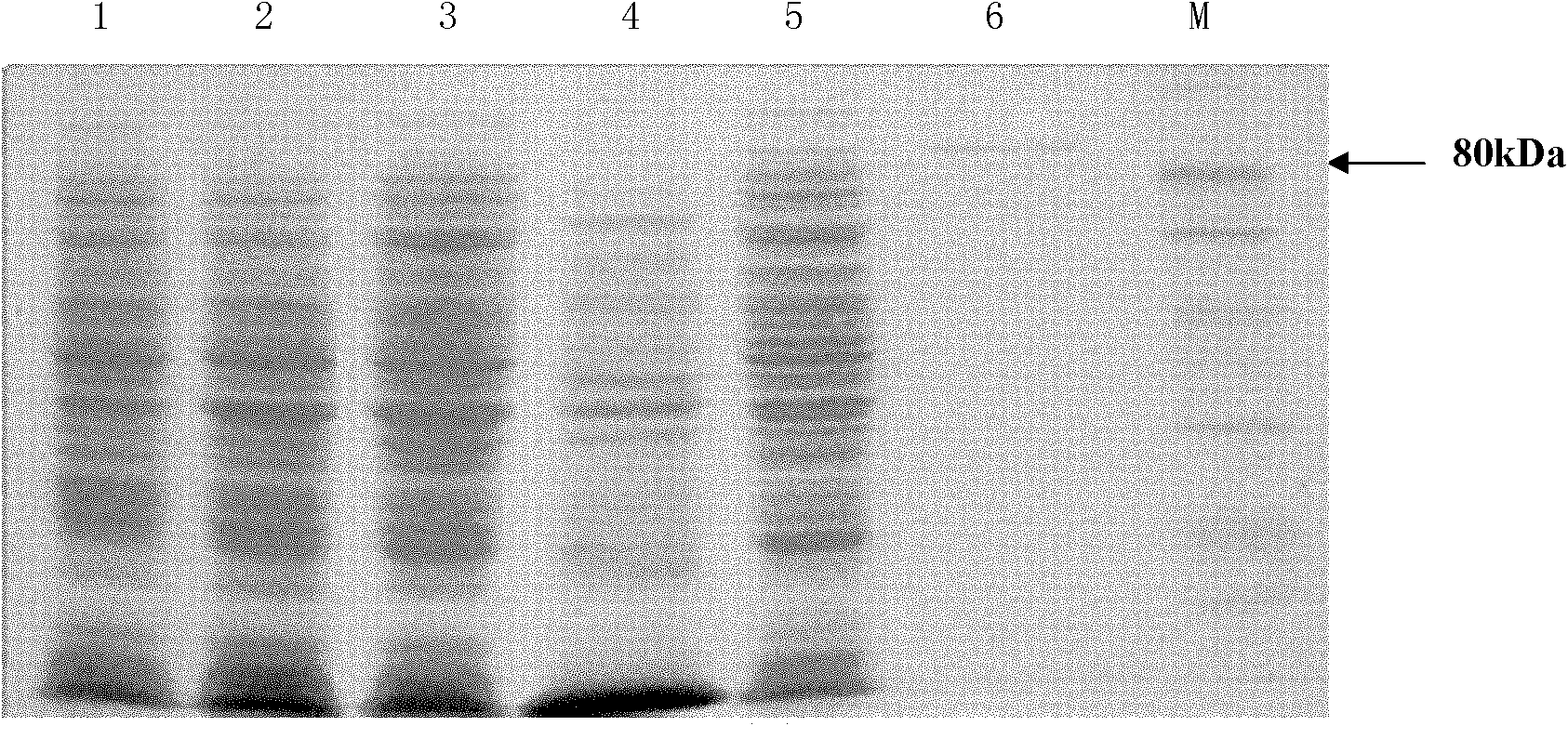
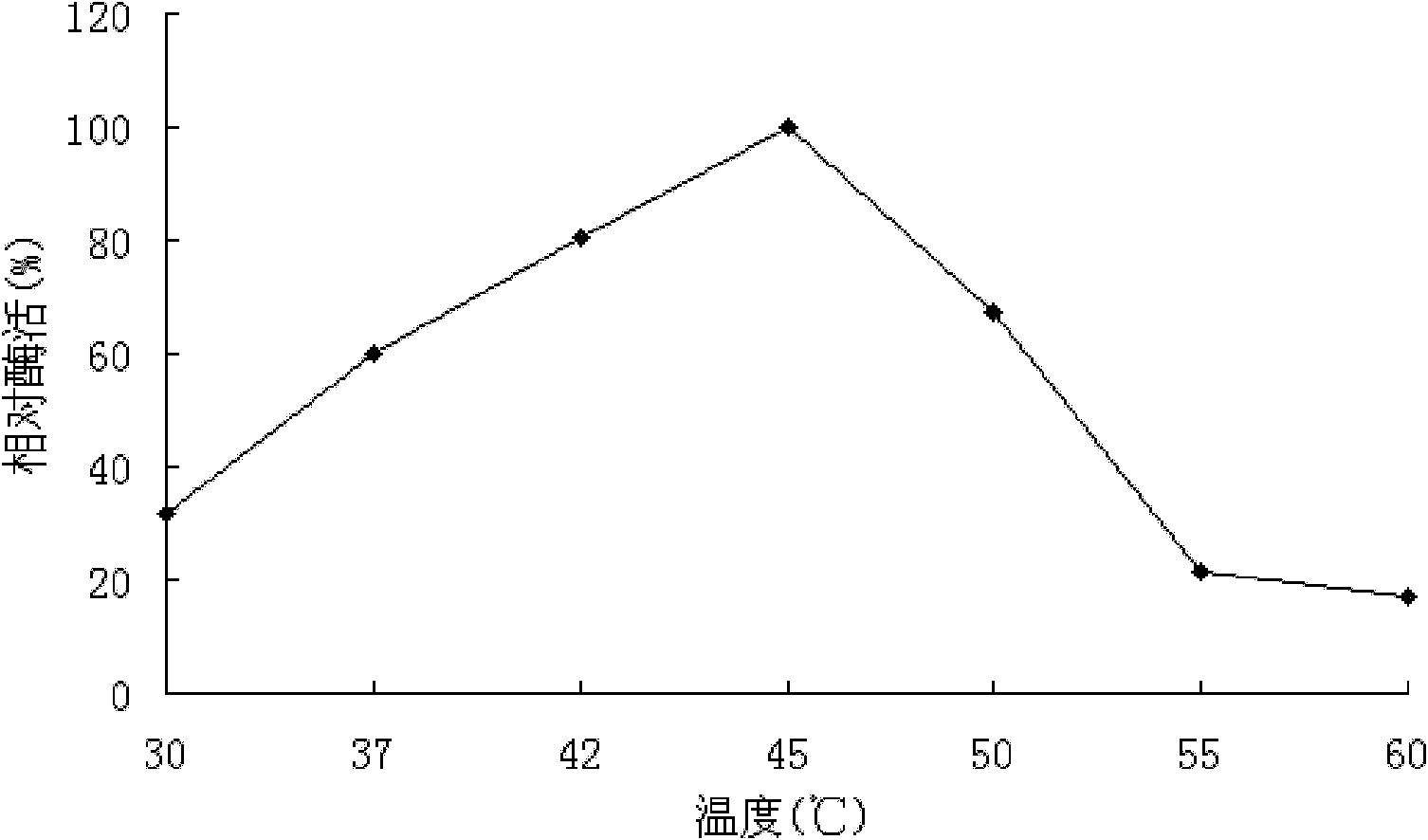
[0154] Embodiment 3, preparation of LGAL protein (genetic engineering expression obtains α-galactosidase)
[0155] The recombinant bacteria R-pET-lgal and the recombinant bacteria R-pET-30a(+) were respectively operated as follows:
[0156] 1. Fermentation of recombinant bacteria
[0157] (1) The recombinant bacteria were inoculated in 10 ml of liquid LB medium (containing 100 μg / mL of kanamycin), cultured on a shaker at 37° C. (250 rpm, amplitude 26 mm) overnight.
[0158] (2) Transfer to 100ml liquid LB medium (containing 100μg / mL kanamycin), 250rpm, amplitude 26mm) to OD by 1% volume 600 0.6 to 0.8.
[0159] (3) Induced expression
[0160] Add IPTG (to make the final concentration 0.4mmol / L), and culture on a shaker at 28°C (250rpm, amplitude 26mm) for 4 hours to obtain 100ml fermentation broth.
[0161] 2. Protein extraction
[0162] (1) Get the 100ml fermented liquid obtained in step 1, and collect the thalline by centrifugation.
[0163](2) Add 20Mm Tris-HCl buffer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com