Modification method for improving high-temperature cycle performance and ionic conductance of lithium iron phosphate material
A lithium iron phosphate, high-temperature cycle technology, applied in the direction of circuits, electrical components, battery electrodes, etc., can solve the problems of difficult diffusion rate of lithium ions and insufficient research on diffusion mechanism, and achieve low heat treatment temperature, stable and controllable product performance, The effect of stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
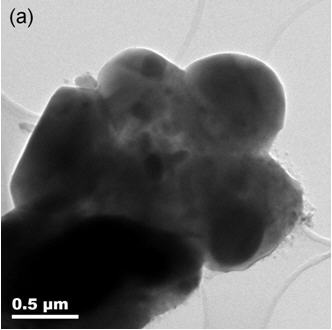
[0018] Example 1: Take an appropriate amount of tetraethyl orthosilicate and absolute ethanol to prepare a 2M solution, then add an appropriate amount of citric acid and polyvinyl alcohol as a chelating agent, and stir continuously at about 50°C to form a sol with a suitable viscosity. Silica accounts for 2wt% of the lithium iron phosphate by adding the lithium iron phosphate powder, continue to add absolute ethanol, and continue to stir, stop adding absolute alcohol after the sample viscosity is moderate, and continue to stir for 2 hours. After stirring evenly, place in a drying oven at 70° C. for heat preservation and drying to obtain the precursor. The precursor was heated up to 500°C at a speed of 5°C / min and kept for 1 hour, cooled to room temperature with the furnace, then crushed and passed through a 200-mesh sieve to obtain the final product. The transmission electron microscope photograph of the obtained product is as follows: figure 1 ,Depend on figure 1 It can be ...
Embodiment 2
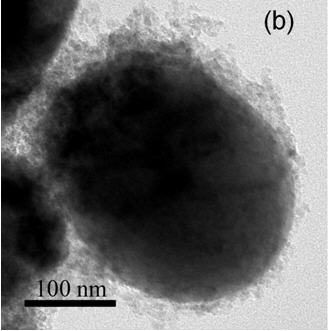
[0019] Example 2: Take an appropriate amount of aluminum isopropoxide and absolute ethanol to prepare a 2M solution, then add an appropriate amount of citric acid and polyvinyl alcohol as a chelating agent, and stir continuously at about 50°C to form a sol with a suitable viscosity. Aluminum accounted for 2wt% of the lithium iron phosphate and added to the lithium iron phosphate powder, continued to add absolute ethanol, and continued to stir, stop adding absolute alcohol after the sample viscosity was moderate, and continued to stir for 2 hours. After stirring evenly, place in a drying oven at 70° C. for heat preservation and drying to obtain the precursor. The precursor was heated up to 500°C at a speed of 5°C / min and kept for 1 hour, cooled to room temperature with the furnace, then crushed and passed through a 200-mesh sieve to obtain the final product. The transmission electron microscope photograph of the obtained product is as follows: figure 2 ,Depend on figure 2 I...
Embodiment 3
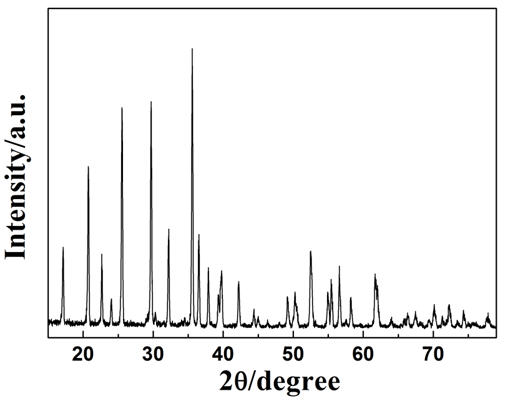
[0022] Example 3: Take an appropriate amount of butyl titanate and absolute ethanol to prepare a 2M solution, then add an appropriate amount of citric acid and polyvinyl alcohol as a chelating agent, and stir continuously at about 50°C to form a sol with a suitable viscosity. Titanium accounted for 2wt% of the lithium iron phosphate was added to the lithium iron phosphate powder, continued to add absolute ethanol, and continued to stir, stop adding absolute alcohol after the sample viscosity was moderate, and continued to stir for 2 hours. After stirring evenly, place in a drying oven at 70° C. for heat preservation and drying to obtain the precursor. The precursor was heated up to 500°C at a speed of 5°C / min and kept for 1 hour, cooled to room temperature with the furnace, then crushed and passed through a 200-mesh sieve to obtain the final product. The transmission electron micrograph of the resulting product shows that the surface of the coated lithium iron phosphate partic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com