Pharmaceutical composition containing cefmetazole sodium compound, and preparation method thereof
A technology of cefmetazole sodium and cefmetazole acid, applied in the field of medicine, can solve the problems of instability of cefmetazole sodium, high side effects and allergic rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
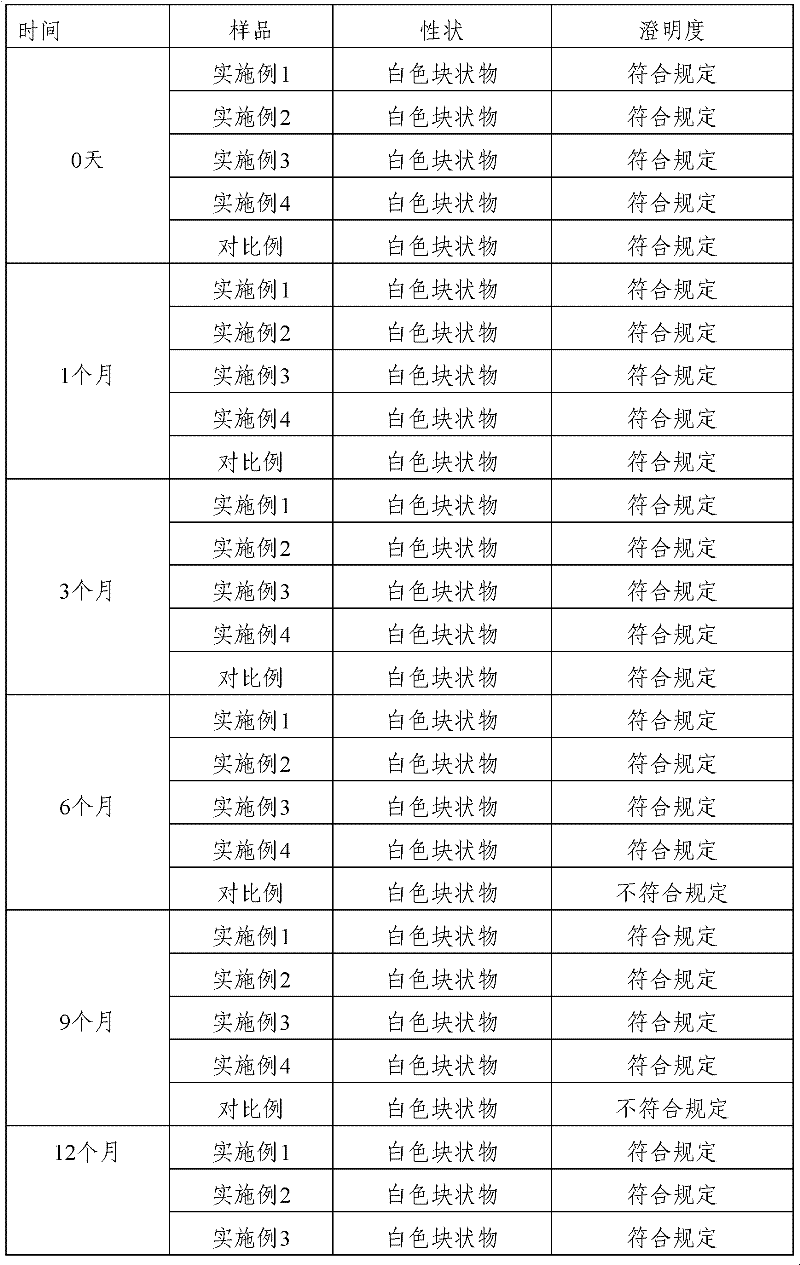
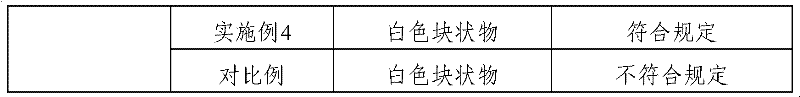
Examples
Embodiment 1
[0018] Prescription: (100 bottles)
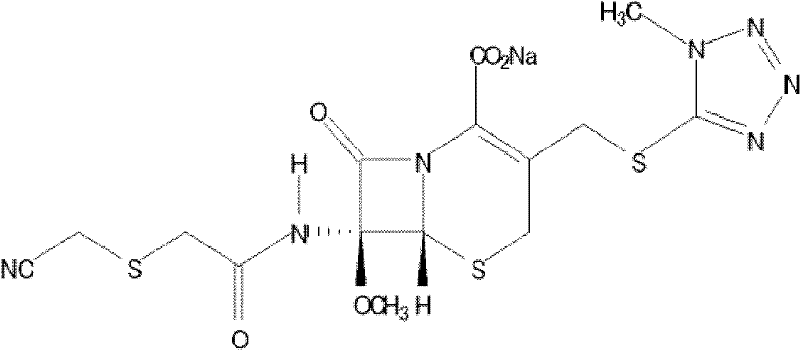
[0019] Cefmetazole Acid 25g
[0021] Mannitol 0.1g
[0022] Preparation Process
[0023] 1) Dissolve the prescribed amount of cefmetazole acid, sodium carbonate and mannitol in water for injection, add water to 1000ml, and stir at 150r / min to make the reaction complete;
[0024] 2) Add activated carbon to the above-mentioned fully reacted solution and stir, adjust the pH to 6 with sodium hydroxide, filter to remove carbon, then filter through a filter membrane, and fill;
[0025] 3) Cool down the above-mentioned filled solution rapidly to freezing in a freeze dryer, maintain freezing at -40°C for 3 hours, vacuumize, slowly heat up to below the eutectic point, and vacuum-dry until the vacuum degree is maintained at 5-10pa Continue to heat up while heating up to 25° C. within 2 hours, and maintain until the degree of vacuum no longer changes to obtain a freeze-dried powder. Example 2
Embodiment 2
[0026] Prescription: (100 bottles)
[0027] Cefmetazole Acid 25g
[0028] Sodium carbonate 2.53g
[0029] Mannitol 0.2g
[0030] 1) Dissolve the prescribed amount of cefmetazole acid, sodium carbonate and mannitol in water for injection, add water to 1000ml, and stir at 190r / min to make the reaction complete;
[0031] 2) Add activated carbon to the above-mentioned fully reacted solution and stir, adjust the pH to 6 with sodium hydroxide, filter to remove carbon, then filter through a filter membrane, and fill;
[0032] 3) Rapidly cool down the above-mentioned filled solution to freezing in a freeze dryer, maintain freezing at -30°C for 5 hours, vacuumize, slowly raise the temperature to below the eutectic point, and vacuum-dry until the vacuum degree is maintained at 5-10pa Continue to heat up while heating up to 30° C. within 4 hours, and maintain until the degree of vacuum no longer changes to obtain a freeze-dried powder.
Embodiment 3
[0034] Prescription: (100 bottles)
[0035] Cefmetazole Acid 25g
[0036] Sodium carbonate 2.53g
[0037] Mannitol 0.3g
[0038] Preparation Process
[0039] 1) Dissolve the prescribed amount of cefmetazole acid, sodium carbonate and mannitol in water for injection, add water to 1000ml, and stir at 160r / min to make a complete reaction;
[0040] 2) Add activated carbon to the above-mentioned fully reacted solution and stir, adjust the pH to 6 with sodium hydroxide, filter to remove carbon, then filter through a filter membrane, and fill;
[0041] 3) Cool down the above-mentioned filled solution rapidly to freezing in a freeze dryer, maintain freezing at -35°C for 4 hours, vacuumize, slowly raise the temperature to below the eutectic point, and vacuum-dry until the vacuum degree is maintained at 5-10pa Continue to heat up while heating up to 30° C. within 3 hours, and maintain until the degree of vacuum no longer changes to obtain a freeze-dried powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com