Application of erythropoietin source peptide to preparation of medicament for treating autoimmune disease of nervous system
An erythropoietin and autoimmune technology, applied in the field of biomedicine, can solve the problems of blood pressure restricting the use of erythropoietin, and achieve the effects of recovery of exercise ability, improvement of limb reflex ability, and excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of Erythropoietin-derived Peptides
[0030] The amino acid sequence of the erythropoietin-derived peptide is as follows: Gln Glu Gln Leu Glu Arg Ala Leu Asn Ser Ser (this segment of polypeptide is derived from the 58th (glutamine) and 62nd (glutamic acid) of erythropoietin in turn. ), 65th (glutamine), 69th (leucine), 72nd (glutamic acid), 76th (arginine), 79th (alanine), 80th (leucine), 83rd (asparagine), 84th (serine), 85th (serine);
[0031]Erythropoietin-derived peptides were synthesized on an ARI 431A solid-phase peptide synthesizer (PE Company, USA). Methods The standard fluorenylmethoxycarbonyl (Fmoc) protocol was used, and arginine was coupled twice. Initially select 0.125mmol p-hydroxymethylphenoxymethyl polystyrene resin (HMP resin), extend the peptide chain from the carboxyl terminal to the amino terminal one by one according to the polypeptide sequence, the amount of each amino acid is 0.5mmol, and the mole of the resin The ratio is...
Embodiment 2
[0033] Example 2 Preparation of Erythropoietin-derived Peptide Liposomes
[0034] Erythropoietin-derived peptide liposomes were prepared by reverse evaporation method. Dissolve lecithin and cholesterol in diethyl ether at a mass ratio of 1:1, distill off the organic solvent therein under reduced pressure, and form a uniform W / O emulsion in an ultrasonic water bath for 5 minutes, then add an appropriate amount such as SEQ ID NO: The erythropoietin-derived peptide shown in 1 was repeatedly frozen and thawed in a 42° water bath and dry ice, and passed through a film extruder with a pore size of 100 nm several times. Utilize that the volume of the erythropoietin-derived peptide shown in SEQ ID NO: 1 increases after binding to the liposome, and the erythropoietin-derived peptide that is not bound to the liposome is separated with an agarose gel CL-4B column. The mass ratio of lecithin, cholesterol and small molecule polypeptide in the liposome is 50:50:1. Then the morphological s...
Embodiment 3
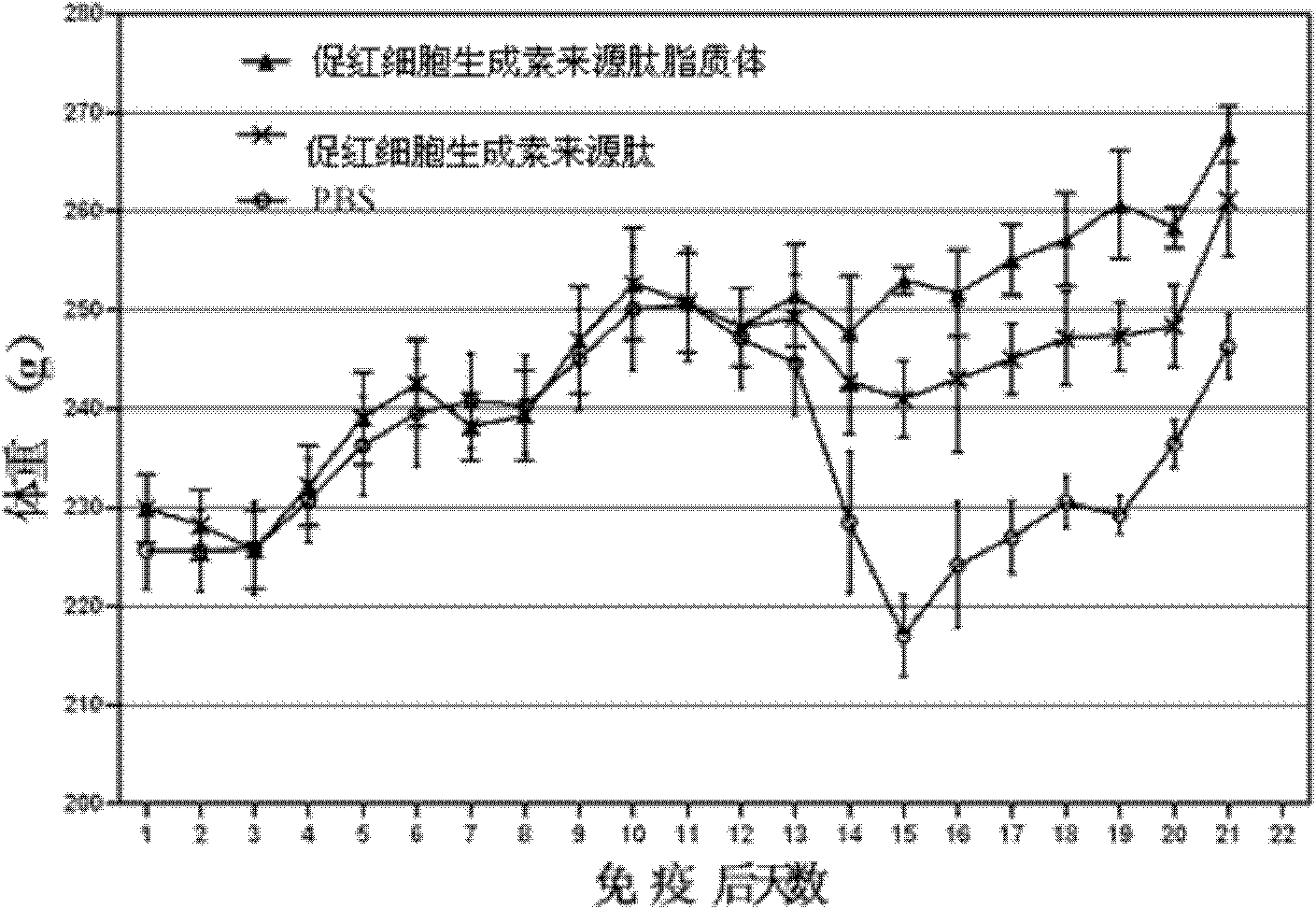
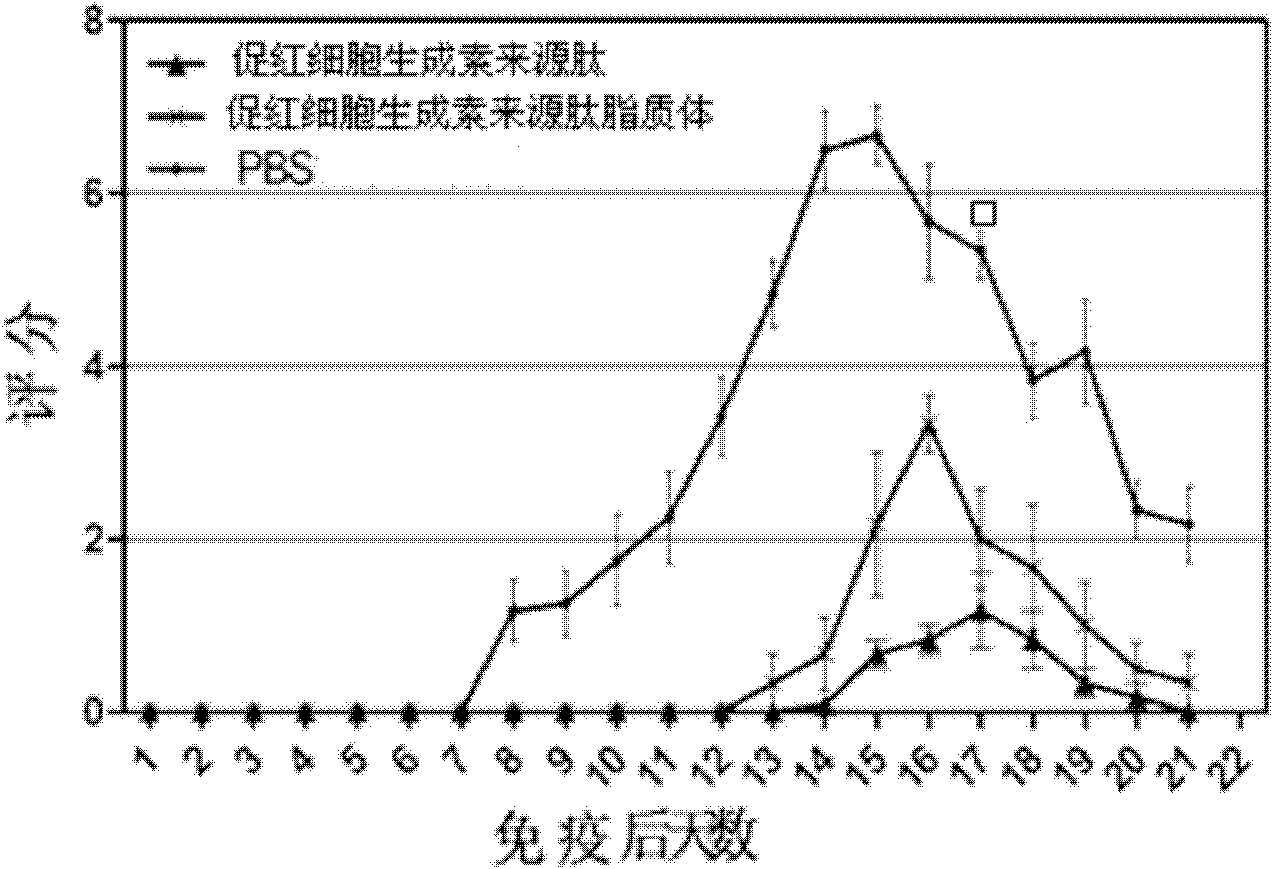
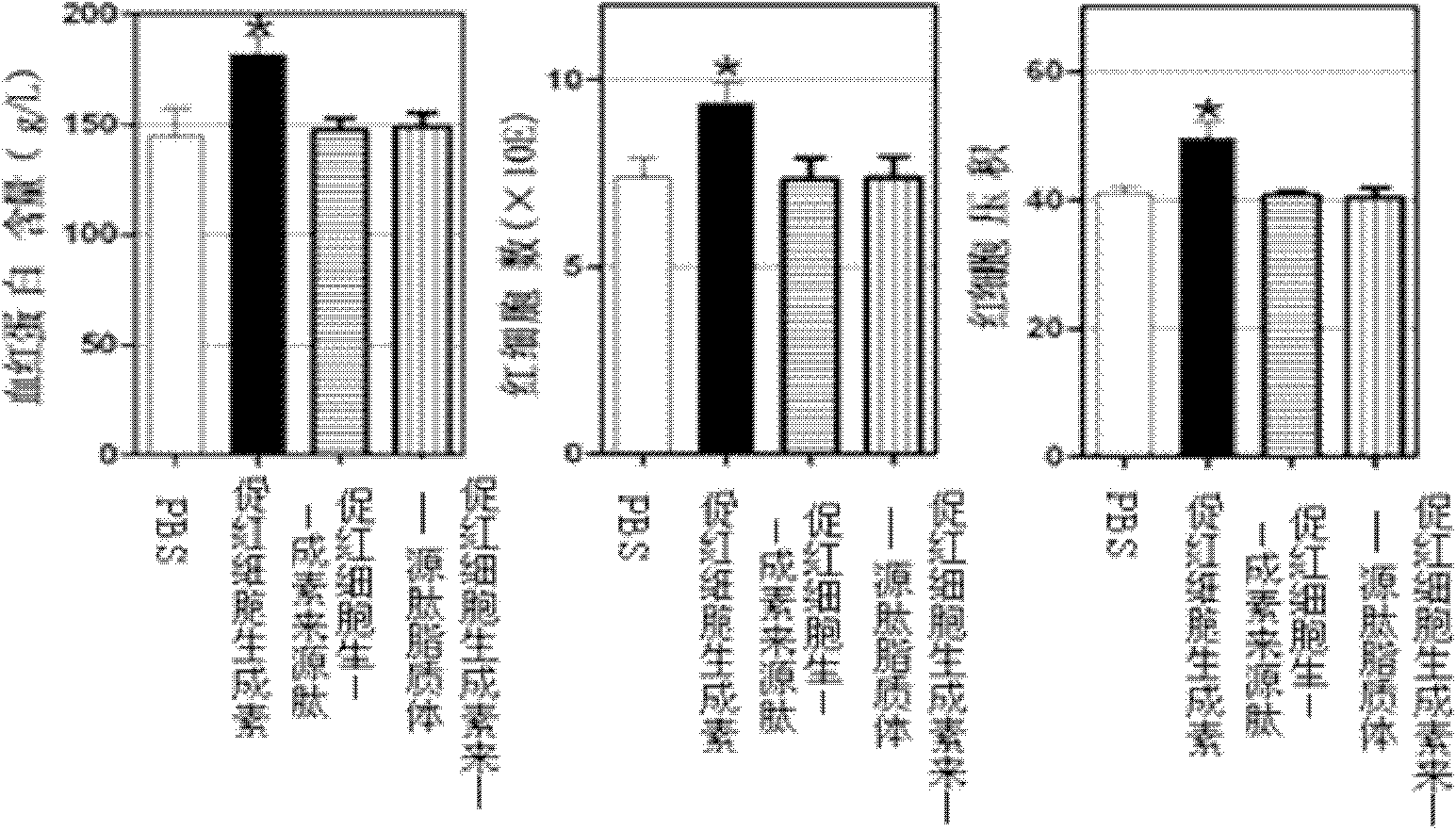
[0035] Example 3 Application of Erythropoietin-derived Peptide and Liposome in Autoimmune Peripheral Neuritis
[0036] 1. Animals:
[0037] Lewis rats, male, 8-10 weeks old, weighing 200-220 grams, were purchased from the Experimental Animal Center of Third Military Medical University, and were normally kept in a clean animal room.
[0038] 2. Drugs:
[0039] The treatment group of erythropoietin-derived peptide liposomes: intraperitoneal injection of erythropoietin-derived peptide liposomes for the treatment of nervous system autoimmune diseases at 20 nmol / kg body weight, a total of 6 animals;
[0040] Erythropoietin-derived peptide treatment group: intraperitoneal injection of 20 nmol / kg body weight of erythropoietin-derived peptide shown in SEQ ID NO: 1 for the treatment of nervous system autoimmune diseases, a total of 6 animals;
[0041] PBS group: intraperitoneal injection of the same volume of PBS as the treatment group, a total of 6 rats.
[0042] 3. Test method:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com