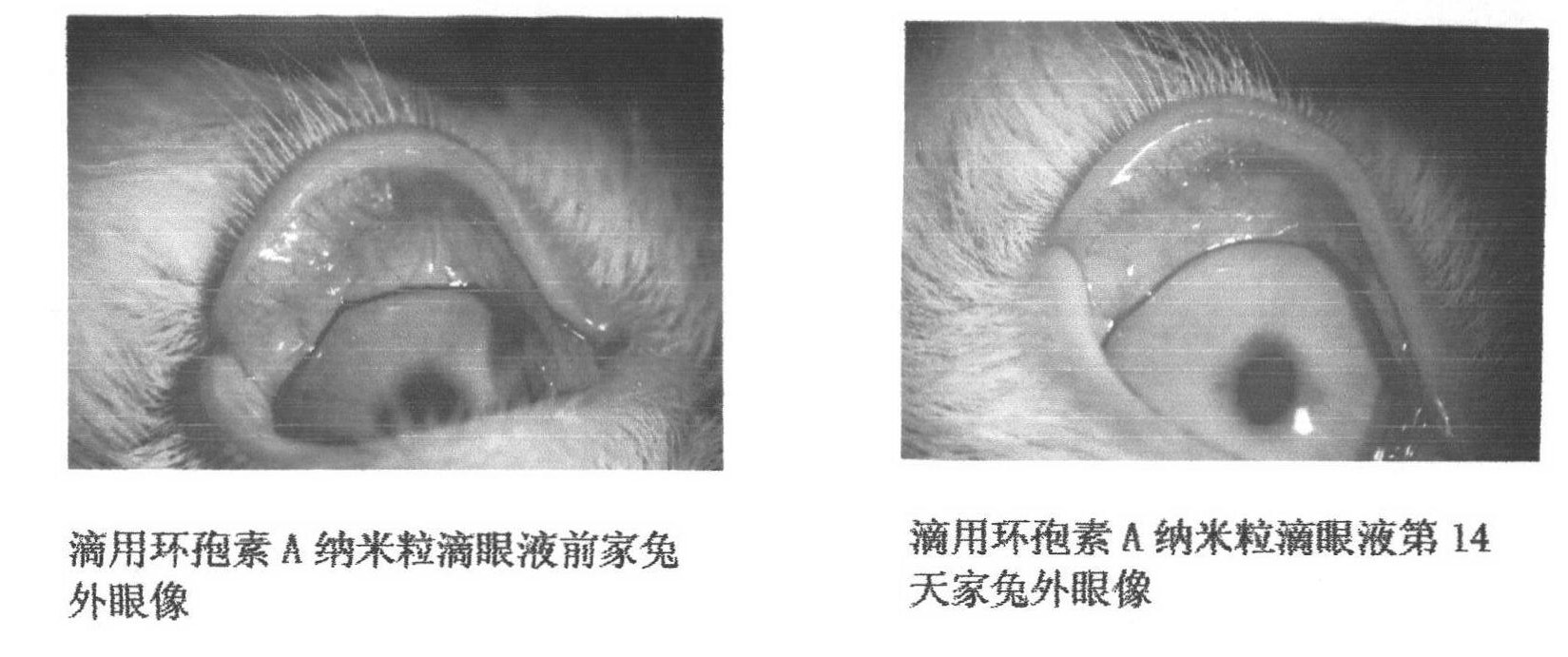
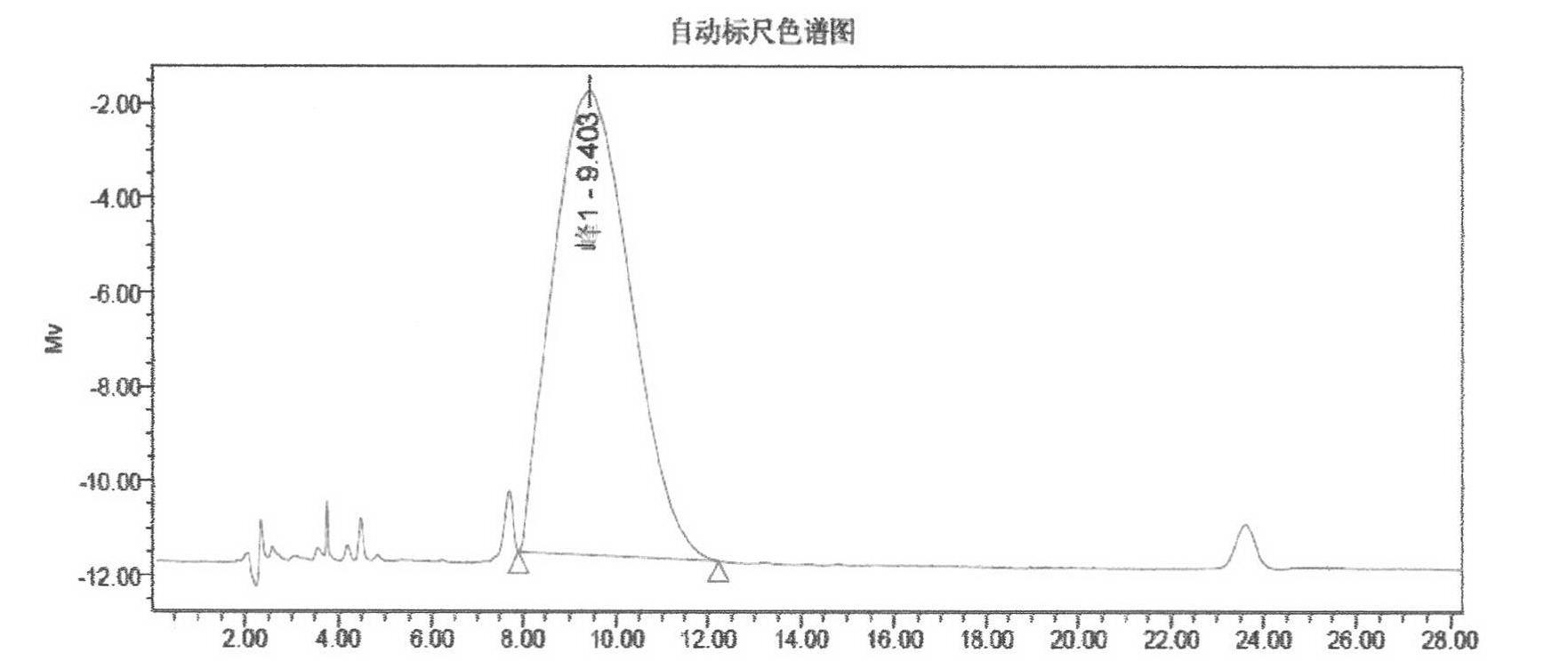
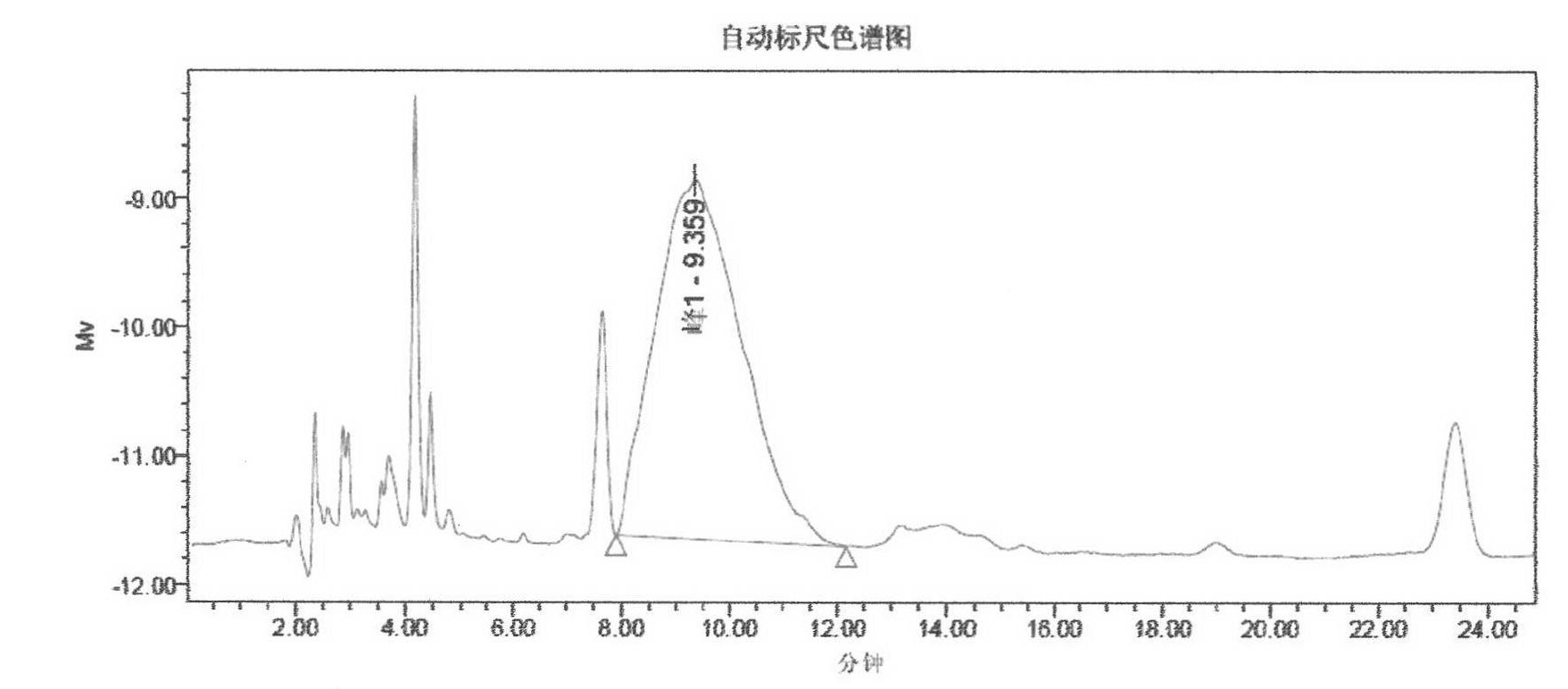
Preparation of cyclosporine A nano-particle eye drop
A technology of cyclosporine and eye drops, which is applied in the directions of inactive medical preparations, cyclic peptide components, sensory diseases, etc., can solve the problems of low bioavailability, poor water solubility, large toxic and side effects, etc. Convenience, reduce toxic and side effects, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0049] Using the emulsified solvent evaporation method, polyethylene glycol-polylactic acid copolymer was used to encapsulate cyclosporine A drug, and spherical nanoparticles with a particle size of about 296.9±32.06nm were obtained, the drug loading was 17.02%, and the encapsulation efficiency is 85.10%. The specific implementation is as follows:
[0050] (1) Dissolve 50mg of polyethylene glycol-polylactic acid copolymer in 6mL of dichloromethane solution, 10mg of cyclosporine A in 0.5mL of dichloromethane, mix the two, and add the mixed solution to the solution containing 0.6% surfactant 20mL water of polyvinyl alcohol (PVA), at room temperature, magnetically stirred for 10min;
[0051] (2) The above solution is mixed with a high-pressure homogenizer to form an emulsion;
[0052] (3) the emulsion removes methylene chloride with a rotary evaporator;
[0053] (4) Centrifuge at 12,500 rpm for 6 minutes, redissolve in normal saline, and obtain cyclosporine A polyethylene glyc...
Embodiment 2
[0057] Using the emulsified solvent evaporation method, the polyethylene glycol-polylactic acid copolymer was used to encapsulate the cyclosporine A drug to obtain spherical nanoparticles with a particle size of about 252.7±27.29nm, the drug loading was 6.98%, and the encapsulation efficiency is 69.80%. The specific implementation is as follows:
[0058] (1) Dissolve 50mg of polyethylene glycol-polylactic acid copolymer in 6mL of dichloromethane solution, 5mg of cyclosporin A in 0.5mL of dichloromethane, mix the two, and add the mixed solution to the solution containing 0.6% surfactant 20mL water of polyvinyl alcohol (PVA), at room temperature, magnetically stirred for 10min;
[0059] (2) The above solution is mixed with a high-pressure homogenizer to form an emulsion;
[0060] (3) the emulsion removes methylene chloride with a rotary evaporator;
[0061] (4) Centrifuge at 12,500 rpm for 6 minutes, redissolve in normal saline, and obtain cyclosporine A polyethylene glycol-p...
Embodiment 3
[0063] Using the nanoprecipitation method, polyethylene glycol-polylactic acid copolymer was used to encapsulate cyclosporine A drug, and spherical nanoparticles with a particle size of about 99.27±18.31nm were obtained, the drug loading was 0.84%, and the encapsulation efficiency 42.26%. The specific implementation is as follows:
[0064] (1) Dissolve 20 mg of polyethylene glycol-polylactic acid copolymer in 3 mL of acetone solution, and 0.4 mg of cyclosporine A in 2 mL of acetone solution as the oil phase;
[0065] (2) Add 5 mL of the oil phase dropwise to 10 mL of mechanically stirred water, and stir for 30 min;
[0066] (3) the emulsion removes the organic solvent with a rotary evaporator;
[0067] (4) Centrifuge at 13,000rpm for 10min, redissolve in normal saline, and obtain cyclosporine A polyethylene glycol-polylactic acid copolymer nano eye drops;
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com