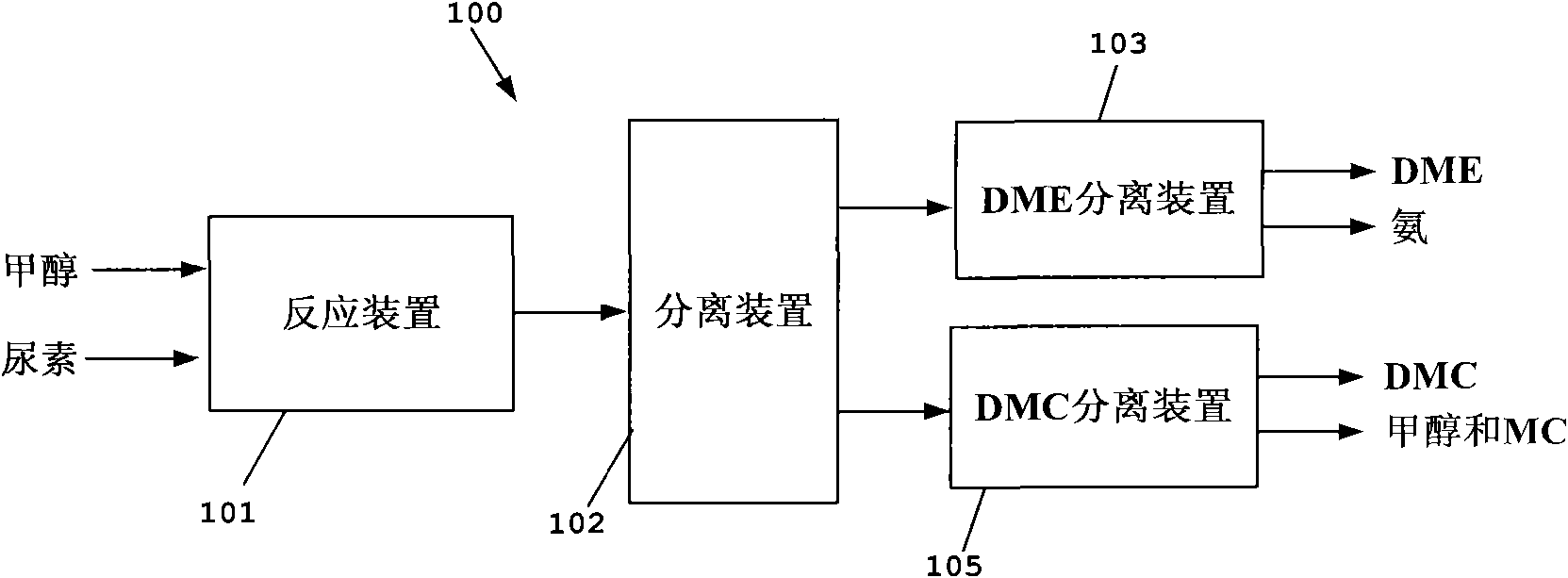
Process for co-producing dimethyl carbonate and dimethyl ether by urea alcoholysis method
A technology of dimethyl carbonate and dimethyl ether, which is applied in the field of co-production of dimethyl carbonate and dimethyl ether by urea alcoholysis, can solve the problems of harsh operating conditions, difficult to remove chloride ions, serious inactivation, etc., and increase production and quality, reasonable economic efficiency, and the effect of no discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
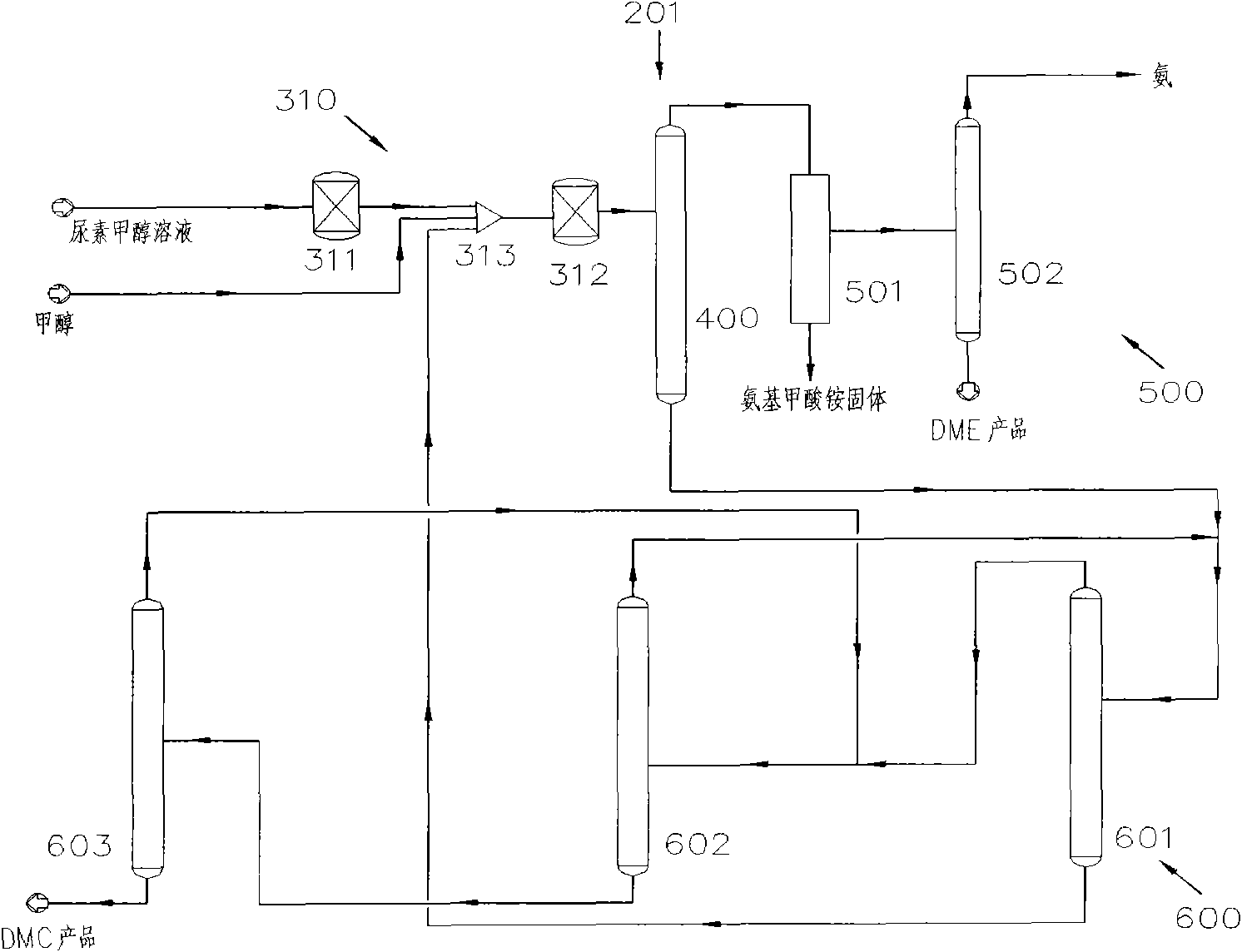
[0055] Such as figure 2 As shown, the system 201 for the co-production of DMC and DME by urea alcoholysis in Embodiment 1 includes a reaction device 310 , a separation device 400 , a DME separation device 500 and a DMC separation device 600 . Wherein, the reaction device 310 includes a first reactor 311 and a second reactor 312; the separation device 400 is a gas-liquid separation tower; the DME separation device 500 includes a solid-liquid separation device 501 and a rectification tower 502; the DMC separation device 600 includes a concentration Column 601, pressurization column 602 and rectification column 603.
[0056] The technical process of the co-production of DMC and DME by urea alcoholysis in embodiment one is as follows:
[0057] Preheat the urea-methanol solution prepared with the molar ratio of methanol and urea as (1-40): 1, or further (1-5): 1, or further as (1-2): 1, and then input it into the first The reaction is carried out in a reactor 311. The first rea...
Embodiment 2
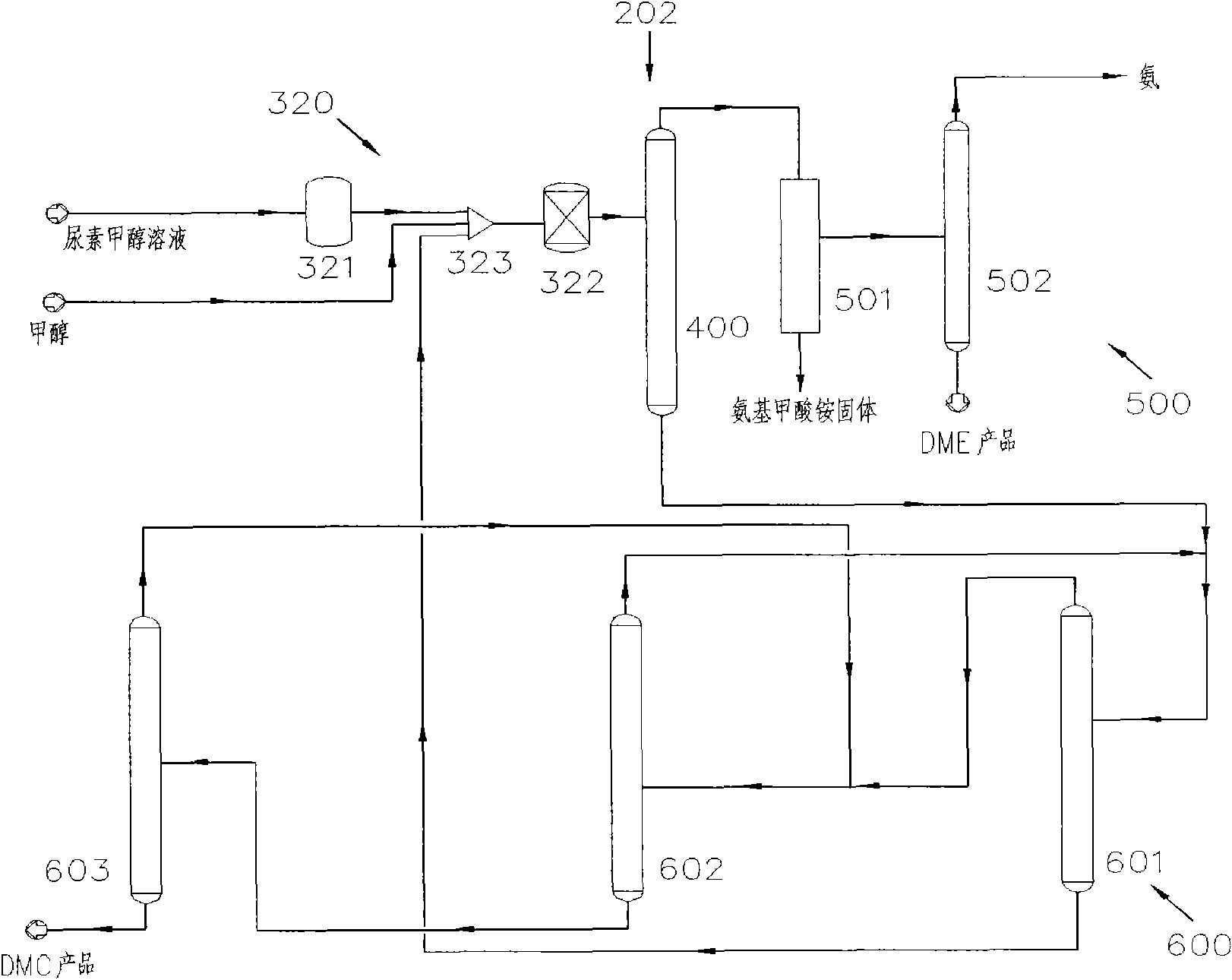
[0068] Such as image 3 As shown, the system 202 for the co-production of DMC and DME by urea alcoholysis in the second embodiment includes a reaction device 320 , a separation device 400 , a DME separation device 500 and a DMC separation device 600 . Wherein, the reaction device 320 includes a first reactor 321 and a second reactor 322, the first reactor 321 is a slurry bed reactor, and the second reactor 322 is a fixed bed reactor. The second reactor 322, the separation device 400, the DME separation device 500 and the DMC separation device 600 are the same or similar to those in the first embodiment.
[0069] The technical process of the co-production of DMC and DME by urea alcoholysis in embodiment two is as follows:
[0070] Preheat the urea-methanol solution prepared with the molar ratio of methanol and urea as (1-40): 1, or further (1-5): 1, or further as (1-2): 1, and then input it into the first The reaction is carried out in a reactor 321. The first reactor 321 is...
Embodiment 3
[0073] Such as Figure 4 As shown, the system 203 for the co-production of DMC and DME by urea alcoholysis in the third embodiment includes a reaction device 330 , a separation device 400 , a DME separation device 500 and a DMC separation device 600 . Wherein, the reaction device 330 includes a two-stage fixed-bed reactor 331, and the separation device 400, the DME separation device 500 and the DMC separation device 600 are the same as those in the first embodiment.
[0074] The technical process of urea alcoholysis co-production DMC and DME in embodiment three is as follows:
[0075] The molar ratio of methanol and urea is (1-40): 1, or further (1-5): 1, or the urea-methanol solution prepared in the ratio of (1-2): 1 and from the concentration tower The output material obtained from the bottom of the tower of 601 and the supplementary methanol delivered from outside are mixed in the mixer 333 and then input into the reactor 331 from the lower part of the reactor 331 for reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com