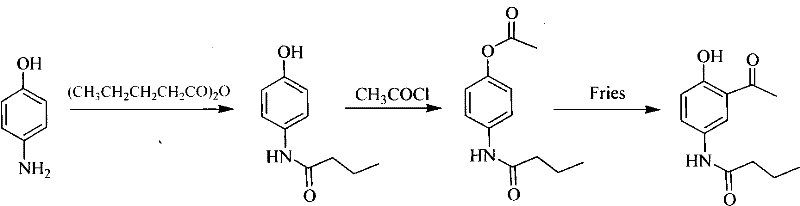
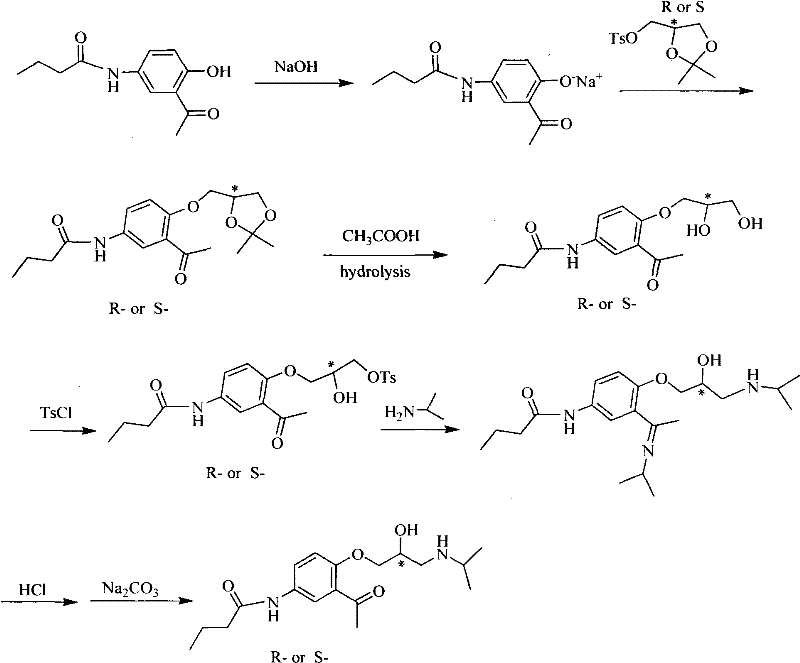
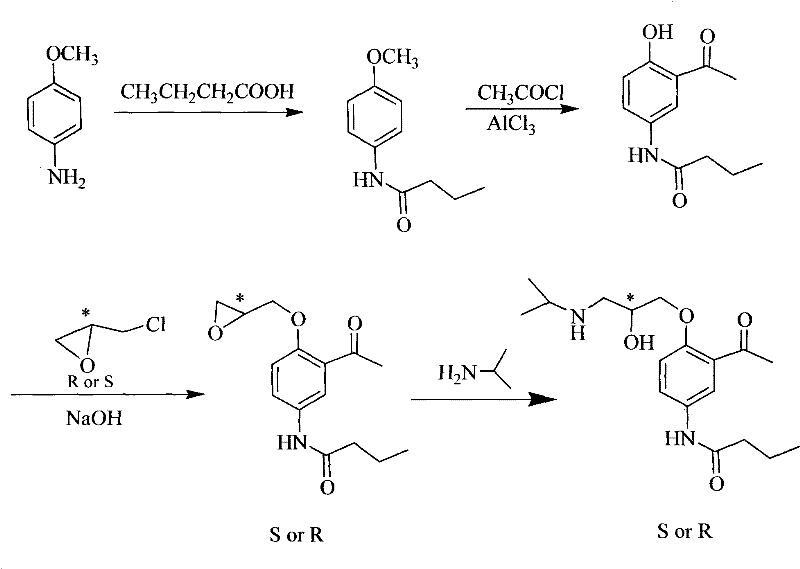
Asymmetrical synthetic method of R-/S-acebutolol
A technology of acebutolol and a synthesis method is applied in the field of asymmetric synthesis of optically pure cardiovascular and cerebrovascular drugs R- or S-acebutolol, and can solve the problems of long reaction steps, high production cost, expensive raw materials and the like, Achieve the effect of few reaction steps, low production cost and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of R-acebutolol
[0045] (1) Synthesis of N-(4-methoxyphenyl)-butanamide
[0046] In a 250ml reaction vessel, dissolve 5.0g of p-aminoanisole in 30ml of toluene, add 15ml of n-butyric acid, heat to 140°C, and stop the reaction after reflux for 6 hours. The excess solvent and n-butyric acid were distilled off while hot, cooled to room temperature, the solid obtained was washed with petroleum ether, and recrystallized in a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of ethyl acetate and petroleum ether of 1:1. 6.28 g of white crystals were obtained, which was determined to be N-(4-methoxyphenyl)-butyramide by nuclear magnetic resonance (400 MHz nuclear magnetic analyzer from Bruker, Switzerland), yield: 80%.
[0047] Mp.86-87°C.
[0048] 1 H NMR (400MHz, CDCl 3 ): δ=1.02(t, J=7.36Hz, 3H, CH 2 CH 3 ), 1.77 (m, 2H, CH 2 CH 2 ), 2.33(t, J=7.36Hz, 2H, CH 2 CH 2 ), 3.79 (s, 3H, OCH 3 ), 6.86 (d, J=8.92Hz, 2H, C 6 h 4 ), 7.05(s,...
Embodiment 2
[0064] Synthesis of R-acebutolol
[0065] (1) Synthesis of N-(4-methoxyphenyl)-butanamide
[0066] In a 250ml reaction vessel, dissolve 10.0g of p-aminoanisole in 60ml of toluene, add 30ml of n-butyric acid, heat to 150°C for reflux reaction, and stop the reaction after 8 hours. The excess solvent and n-butyric acid were distilled off while hot, cooled to room temperature, the solid obtained was washed with petroleum ether, and recrystallized in a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of ethyl acetate and petroleum ether of 1:1. 13.5 g of white crystals were obtained, which was determined to be N-(4-methoxyphenyl)-butyramide by nuclear magnetic resonance (400 MHz nuclear magnetic analyzer from Bruker, Switzerland), with a yield of 86%.
[0067] Mp.87-88°C.
[0068] 1 H NMR (400MHz, CDCl 3 ): δ=1.02(t, J=7.36Hz, 3H, CH 2 CH 3 ), 1.77 (m, 2H, CH 2 CH 2 ), 2.33(t, J=7.36Hz, 2H, CH 2 CH 2 ), 3.79 (s, 3H, OCH 3 ), 6.86 (d, J=8.92Hz, 2H, C...
Embodiment 3
[0083] Synthesis of R-acebutolol
[0084] (1) Synthesis of N-(4-methoxyphenyl)-butanamide
[0085] In a 250 ml reaction vessel, dissolve 5.0 g of p-aminoanisole in 50 ml of toluene, add 18 ml of n-butyric acid, heat to 130° C., and stop the reaction after reflux for 9 hours. The excess solvent and n-butyric acid were distilled off while hot, cooled to room temperature, the solid obtained was washed with petroleum ether, and recrystallized in a mixed solvent of ethyl acetate and petroleum ether with a volume ratio of ethyl acetate and petroleum ether of 1:1. 6.24 g of white crystals were obtained, which was determined to be N-(4-methoxyphenyl)-butyramide by nuclear magnetic resonance (400 MHz nuclear magnetic analyzer from Bruker, Switzerland), yield: 80%.
[0086] Mp.86-87°C.
[0087] 1 H NMR (400MHz, CDCl 3 ): δ=1.02(t, J=7.36Hz, 3H, CH 2 CH 3 ), 1.77 (m, 2H, CH 2 CH 2 ), 2.33(t, J=7.36Hz, 2H, CH 2 CH 2 ), 3.79 (s, 3H, OCH 3 ), 6.86 (d, J=8.92Hz, 2H, C 6 h 4 ), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com