Solid-phase synthesis method of vMIP-II (viral macrophage inflammatory protein-II) protein
A solid-phase synthesis and protein technology, which is applied to peptide preparation methods, chemical instruments and methods, viral peptides, etc., can solve the problems of long purification cycle, industrial production and product application limitations, and high preparation cost, and achieve shortened production cycle, Improve the purity and synthesis efficiency of the target peptide, and improve the effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
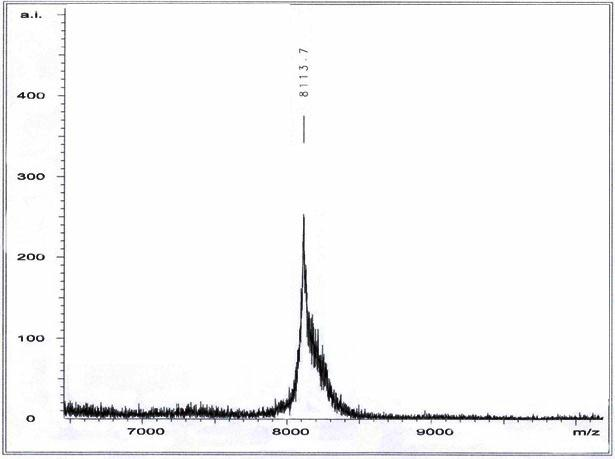
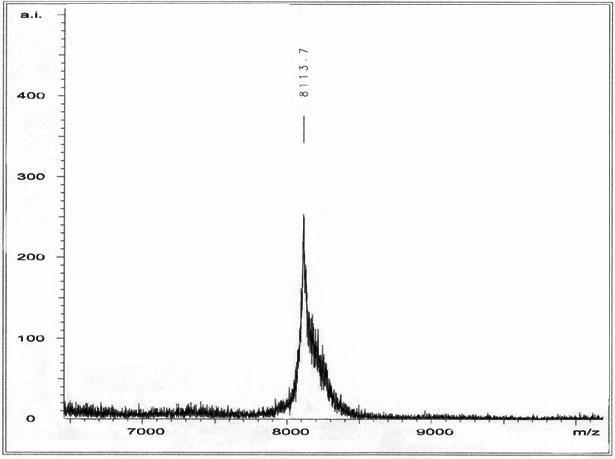
[0019] Example 1 vMIP- II Peptide Synthesis and Purification
[0020] (1) Synthesis of vMIP-II polypeptide
[0021] Polypeptides were synthesized on a peptide synthesizer by Fmoc-solid-phase synthesis. After the reaction bottle was fully cleaned with 100% ethanol, about 40ml of 5% dimethyldichlorosilane was added and placed in a fume hood overnight to silanize the reaction bottle. After roughly washing with ethanol, place the reaction bottle in a -80°C refrigerator for 30 minutes to cool, wash with ethanol again, and dry it for later use; weigh 0.5mmol Wang's resin (Wang's resin) 0.7130g into the reaction bottle, add 3 times the volume of the resin DCM (dichloromethane) solution, swell for 30min and drain the liquid. Then, on the polypeptide synthesizer, it is carried out from the C-terminal to the N-terminal according to the cycle of coupling amino acid, ninhydrin reaction detection and α-amino deprotection until all amino acid residues are coupled. After the coupling i...
Embodiment 2
[0026] Example 2 Determination of polypeptide activity - vMIP-II to HIV-1 ⅢB Inhibition of induced cellular syncytia formation
[0027] Logarithmic growth phase MT 4 Cells (provided by the Guangdong Provincial Center for Disease Control and Prevention) were adjusted to 8×10 per ml with RPMI-1640 medium 6 Take 1ml centrifuge to discard the supernatant, add 500μl cell culture medium to resuspend the cells. Add 80μl MT to each well of a 96-well plate 4 Cell suspension, add HIV-1 ⅢB Viruses (Human HIV-1 ⅢB type) (provided by the Guangdong Provincial Center for Disease Control and Prevention) [approximately 1000 TCID 50 / (10 6 cell)] 20 μl, add 100 μl of different concentrations of vMIP-II (600, 60, 6 ng / ml) in different wells, and set 3 replicate wells for each concentration (the wells with vMIP-II are experimental wells).
[0028] At the same time, a blank control group and a positive control group were set up. Only normal MT was added to the blank control group 4 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com