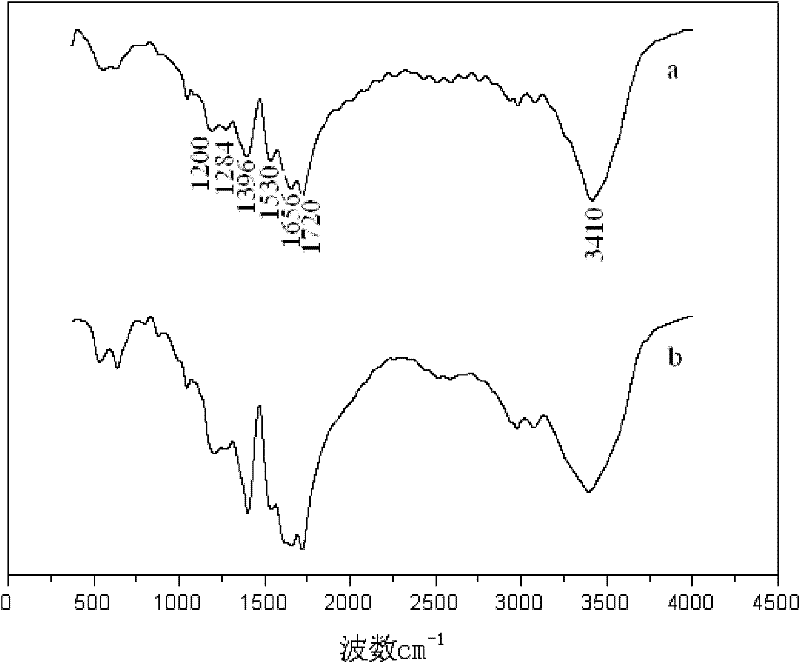
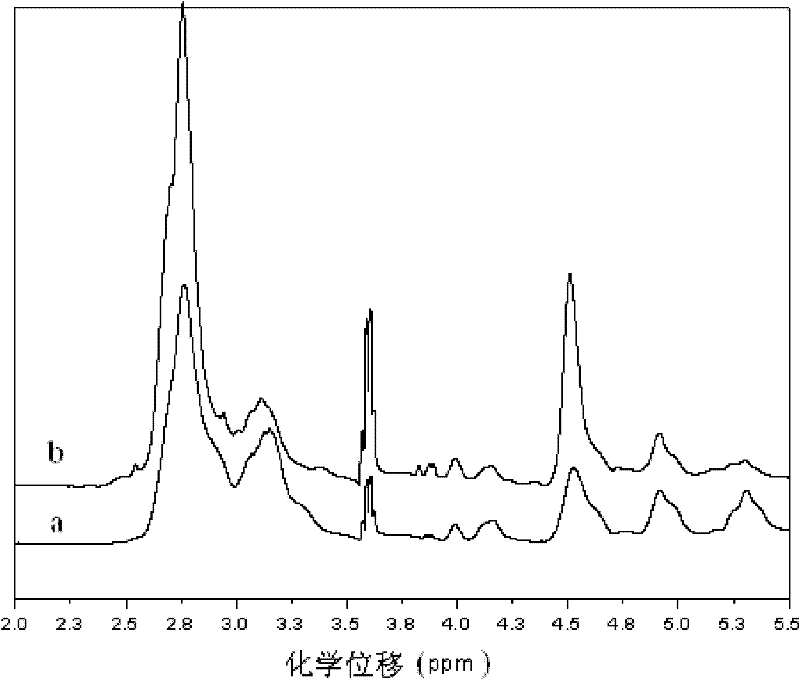
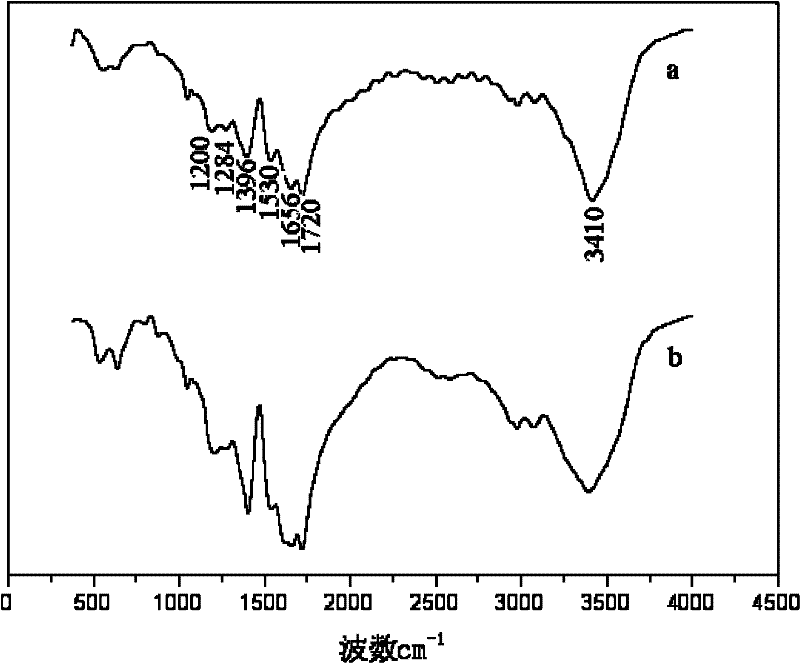
Method for catalytic synthesis of polyaspartic acid by using imidazole type ionic liquid
A technology of polyaspartic acid and ionic liquids, applied in chemical instruments and methods, descaling and water softening, bulk chemical production, etc., can solve problems such as difficult product separation process, high reaction temperature, and low yield. To achieve the effect of facilitating mass transfer and heat transfer, simple processing process, and enhanced thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0008] Specific embodiment one: a kind of method of utilizing imidazoles ionic liquid to catalyze the synthesis of polyaspartic acid of the present embodiment is carried out in the following steps: one, the mass ratio by imidazoles ionic liquid and L-aspartic acid is 1: Weigh the imidazole ionic liquid and L-aspartic acid at a ratio of 0.25 to 1.5, add them into a container with a stirring device, and raise the temperature to 145°C to 158°C under stirring conditions and keep it for 0.5h to 6h to obtain polysuccinyl A mixed liquid of imine and ionic liquid; 2. Cool the mixed liquid of polysuccinimide and ionic liquid obtained in step 1 to 15°C to 30°C, add deionized water under stirring, and solids are precipitated, pumped The solid is separated by filtration, and then washed with deionized water to obtain polysuccinimide; 3. The polysuccinimide obtained in step 4 is completely dissolved with an aqueous sodium hydroxide solution with a mass concentration of 10% to 50%, and then ...
specific Embodiment approach 2
[0011] Embodiment 2: This embodiment differs from Embodiment 1 in that: in step 1, the mass ratio of imidazole-based ionic liquid to L-aspartic acid is 1:0.30-1.2. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0012] Embodiment 3: The difference between this embodiment and Embodiment 1 is that the mass ratio of imidazole-based ionic liquid to L-aspartic acid in step 1 is 1:0.8. Others are the same as in the first embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| scale inhibition rate | aaaaa | aaaaa |
| scale inhibition rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com