Release mass transfer rate adjustable intelligent drug delivery carrier and preparation method thereof
A technology of drug delivery carrier and rate, which is applied in the field of drug delivery carrier and its preparation relying on external environmental stimuli to realize the controllable speed-adjusted release of drugs. Effects of avoiding toxic and side effects, regulating drug release rate, and reducing mass transfer resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The processing steps of the present embodiment are as follows:
[0048] (1) Preparation of thermosensitive submicrospheres
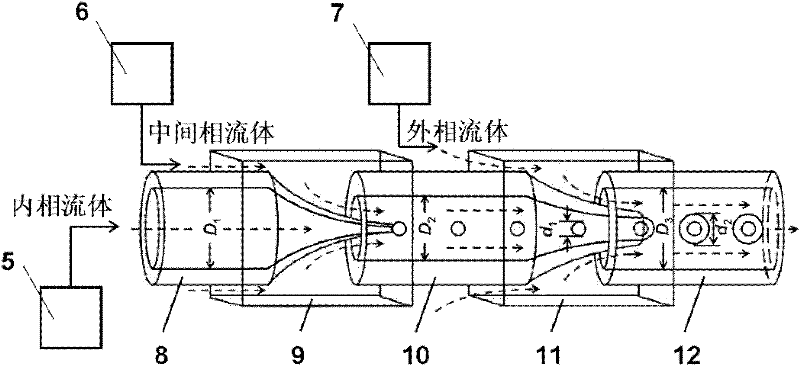
[0049] A device for preparing thermosensitive submicrospheres such as Figure 5 As shown, it comprises a three-necked glass flask 20, a constant temperature circulating water bath 21, and a magnetic stirrer 23. One port of the three-necked glass flask 20 is connected to a spherical condenser tube 18, and the remaining two ports are respectively inserted with a nitrogen-through glass tube 19, and the three-necked glass flask 20 A magnetic stirrer 22 is arranged inside;
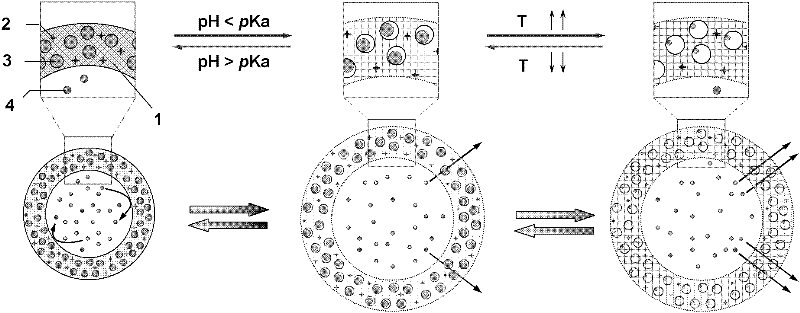
[0050] Add monomer N-isopropylacrylamide, acrylamide and allylamine, cross-linking agent N, N'-methylenebisacrylamide, initiator potassium persulfate to deionized water at room temperature and normal pressure to form a mixed solution , in the mixed solution, the concentration of N-isopropylacrylamide is 0.07mol / L, the molar ratio of acrylamide to N-isopropylacrylamide is 0.2:1, a...
Embodiment 2
[0066] The processing steps of the present embodiment are as follows:
[0067] (1) Preparation of thermosensitive submicrospheres
[0068] The device for preparing temperature-sensitive submicrospheres is the same as in Example 1. Add monomer N-isopropylacrylamide, acrylamide and allylamine, cross-linking agent N, N'-methylene bisacrylamide, initiator ammonium persulfate to deionized water at room temperature and normal pressure to form a mixed solution , in the mixed solution, the concentration of N-isopropylacrylamide is 0.1mol / L, the molar ratio of acrylamide to N-isopropylacrylamide is 0.025:1, allylamine and N-isopropylacrylamide The molar ratio of the ammonium persulfate is 0.025:1, the concentration of ammonium persulfate is 0.25g / L, the molar ratio of N,N'-methylenebisacrylamide and N-isopropylacrylamide is 0.05:1,
[0069] Add the mixed solution into a three-necked glass flask 20, raise the temperature of the mixed solution to 60° C. in a nitrogen atmosphere, and re...
Embodiment 3
[0080] The processing steps of the present embodiment are as follows:
[0081] (1) Preparation of thermosensitive submicrospheres
[0082] The device for preparing temperature-sensitive submicrospheres is the same as in Example 1. Add monomer N-isopropylacrylamide, cross-linking agent N,N'-methylenebisacrylamide, initiator azobisisobutylamidine hydrochloride to deionized water at room temperature and normal pressure to form a mixed solution , in the mixed solution, the concentration of N-isopropylacrylamide is 0.05mol / L, the concentration of azobisisobutylamidine hydrochloride is 0.4g / L, and N,N'-methylenebisacrylamide The molar ratio with N-isopropylacrylamide is 0.1:1,
[0083] Add the mixed solution into a three-necked glass flask 20, raise the temperature of the mixed solution to 70°C in a nitrogen atmosphere and react at this temperature for 5 hours. After the reaction, the reaction solution is centrifugally washed with deionized water to obtain poly( N-isopropylacryla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com