Oral slow release influenza vaccine and preparation method thereof
A vaccine and influenza technology, applied in the field of bioengineering, can solve problems such as stimulating secretory antibodies, and achieve the effects of reducing costs, low cost, and easy storage and transportation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
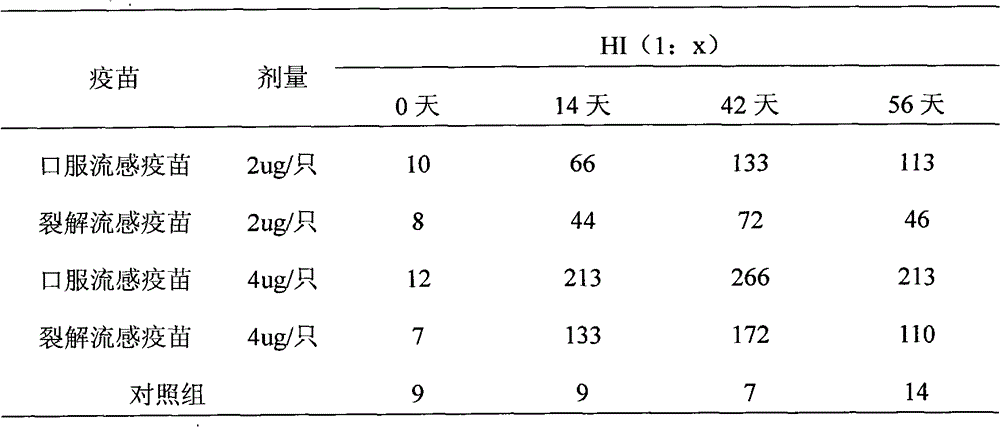
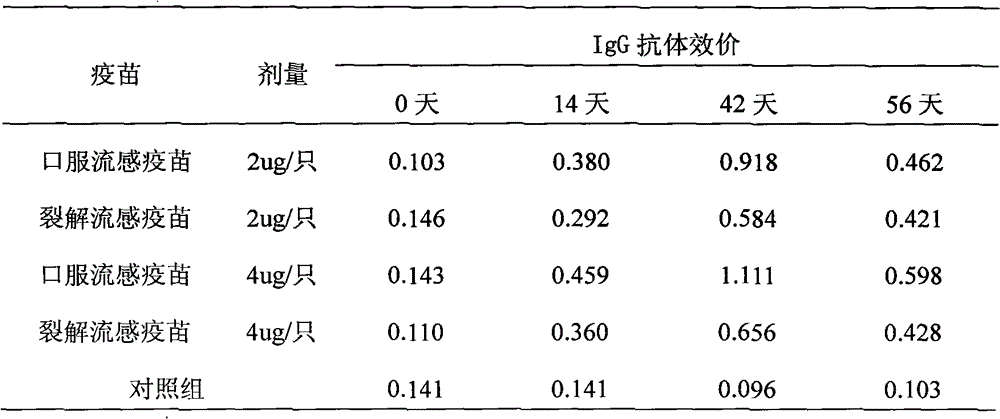
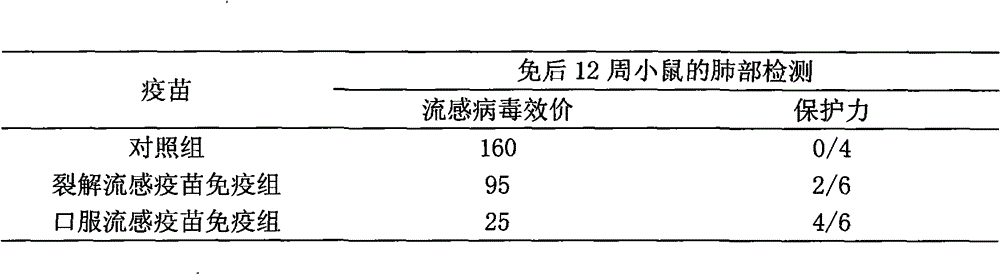
[0032] The oral influenza vaccine buccal tablet involved in the present invention comprises three kinds of existing influenza whole virus inactivated vaccines, split vaccines and subunit vaccines, and its preparation process is as follows:
[0033] (1) Preparation of vaccine seed bank: Like other influenza vaccines of various dosage forms, a three-level seed bank of primary generation, primary generation, and working generation should be established. The three-level seed bank must be verified: hemagglutination titer, virus titer , Sterility test, mycoplasma, identification test, exogenous factors (avian adenovirus, leukemia virus detection) and other items.
[0034](2) Preparation of virus liquid: inoculate chicken embryos with working seeds, inactivate after cultivating, harvesting virus liquid, clarifying and filtering, and make whole virus liquid, subject to verification: hemagglutination titer, sterility test, inactivation Tests, identification tests, etc. Then proceed to...
Embodiment 2
[0058] Prepare the oral vaccine core of freeze-drying, first the influenza vaccine liquid (whole virus inactivation liquid, lysate or subunit vaccine liquid) that reaches vaccine testing requirements is added adjuvant or antacid, choose calcium carbonate in this example, make it The final concentration reaches 60-80 mg / dose; then add a lyophilized stabilizer to properly dilute the vaccine stock solution, for example, to 30-40 μg / dose or 0.3-0.4ml / dose. Available stabilizers include sucrose, dextran or 4% The amino acid is transferred to the blister-like hole of the plastic according to the dosage of 0.5-0.6ml by aseptic filling operation, and after the composition is freeze-dried, the blister-like hole is sealed by a heat sealing method. In addition to the above-mentioned reagents, the lyophilized preparation can also include the following standard components to achieve the purpose of rapid dissolution in the mouth, including: magnesium stearate, carboxymethyl cellulose, hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com