Chicken defensin 9 gene expression vector and preparation method and use thereof
A chicken defensin and gene expression technology, applied in the field of genetic engineering, can solve the problems of lack of drugs and high cost of chemically synthesized antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Cloning of chicken defensin 9 gene
[0043] Use primer sequence SEQ ID NO:1~4 to carry out PCR according to the following procedures: primers (20μmol / L) each 1μL dNTPs (10mmol / L) 1μL, 10×buffer(Mg 2+ ) 5μL, rTaq DNA polymerase (2.5U / μL) 1μL, ddH 2 Add O to 50 μL. Use the obtained PCR product as a template, and use SEQ ID NO:1 and SEQ ID NO:5 as primers for the second round of PCR amplification. The composition of the PCR reaction system is as follows: 10×buffer 5 μL, dNTPs 2 μL, primers (20 μmol / L) each 2 μL, rTaq DNA polymerase (2.5U / μL) 0.5 μL, template 1 μL, ddH 2 O make up to 50μL. PCR reaction program: 94°C pre-denaturation for 2min, 1 cycle; 94°C for 30s, 52°C for 30s, 72°C for 40s, 30 cycles; 72°C after extension for 5min.
[0044] When PCR products are detected by 1.5% agarose gel electrophoresis, specific bands ranging from 100bp to 250bp can be observed, see Figure 4 .
Embodiment 2
[0045] Example 2 Amplification of ov sequence
[0046] The total DNA of the chicken oviduct was used as a template, and the ov sequence fragment was amplified with specific primers SEQ ID NO: 6-7. The composition of the PCR reaction system is as follows: 10×buffer 2.5 μL, dNTPs 2 μL, primers (20 μmol / L) each 1 μL, rTaq DNA polymerase (2.5U / μL) 0.5 μL, template 0.5 μL, ddH 2 O make up to 25μL. PCR reaction program: 94°C pre-change 5min, 1 cycle; 94°C 40s, 55°C 40s, 72°C 80s, 72°C, 30 cycles; 72°C extension 10min. Using the above product as a template, perform another PCR, double the amount of each item added to the system, and the total volume is 50 μL.
[0047] When the PCR product is detected by 1.5% agarose gel electrophoresis, a specific band of about 1000 bp can be observed, see Figure 5 .
Embodiment 3
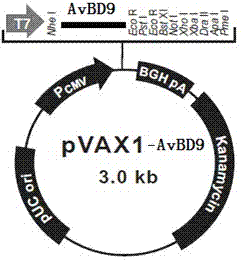
[0048] Example 3 Construction of recombinant eukaryotic expression vector pVAX1-AvBD9
[0049] Restriction enzymes for AvBD9 gene Nhe I and EcoR I Carry out restriction digestion, and act at 37°C for 3 hours. The reaction system is as follows: 10×buffer M 5μL, Nhe I 1μL, EcoR I 1μL, PCR product 25μL, ddH 2 Make up O to 50μL, and use the same endonuclease for pVAX1 plasmid (invitrogen) Nhe I and EcoR I for enzyme digestion. After 3h at 37℃, the reaction system is as follows: 10×buffer M 5μL, Nhe I 1μL, EcoR I 1μL, PCR product 20μL, ddH 2 Make up to 50μL of O and dephosphorylate with CIAP (alkaline phosphatase). All the digested reaction solution was electrophoresed with 1.5% agarose, and the band of the target fragment was cut under ultraviolet light, and the target DNA fragment after digestion was recovered with a gel recovery kit. Under the action of T4 ligase, connect the digested PCR product of AvBD9 gene and digested pVAX1 plasmid, ligate overnight at 4°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com