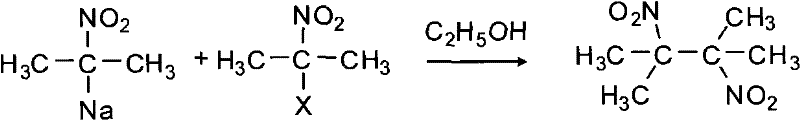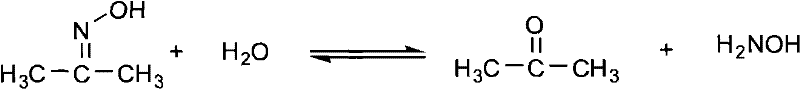The synthetic method of 2,3-dimethyl-2,3 dinitrobutane
A technology of dinitrobutane and a synthesis method, which is applied in the field of compound synthesis, can solve the problems of safety production hazards, increased synthesis cost, poor product quality and the like, and achieves the effects of low production cost, low price and few impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 73g of water to a 250mL three-necked flask equipped with a thermometer, a constant pressure funnel, and an electric stirring device, then add 29.2g (0.40mol) of acetone oxime and 2.9g of modified TS-1, and vigorously stir for 0.5h under electric stirring; Then the water bath is warmed up to 85°C, and 113.3g (1.00mol) of 30% hydrogen peroxide and 30% sodium hydroxide are added dropwise at 85-90°C simultaneously to keep the pH of the reaction system at 9-10, and the dropwise addition is completed at 90°C Keep it warm for 0.5h, then cool to room temperature, and remove the modified TS-1 by centrifugation at 3000rpm. The modified TS-1 is washed twice with 6g of acetone. After the washed acetone is evaporated to dryness, the residue is combined into the mother liquor, and then At 80°C and 0.05 MPa, the mother liquor was decompressed and rotary-evaporated to remove acetone (quantified by GC, 0.32 mol), then cooled to room temperature, filtered, and washed with water to obt...
Embodiment 2
[0040] The consumption of solvent water is changed into 146g, and other is with embodiment 1, and the result is: 2,3-dimethyl-2, the molar yield of 3-dinitrobutane is 12.5%, fusing point is 210.5~212.1 ℃, acetone 0.33 mol, acetone oxime 0.01mol.
Embodiment 3
[0042]The amount of modified TS-1 is changed to 4.3g, and the others are the same as in Example 1. The result is: the molar yield of 2,3-dimethyl-2,3-dinitrobutane is 11.4%, and the melting point is 210.2~212.0°C , acetone 0.34mol, acetone oxime trace.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com