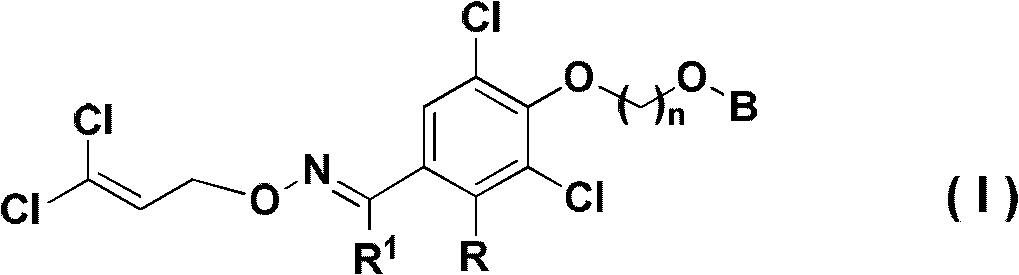
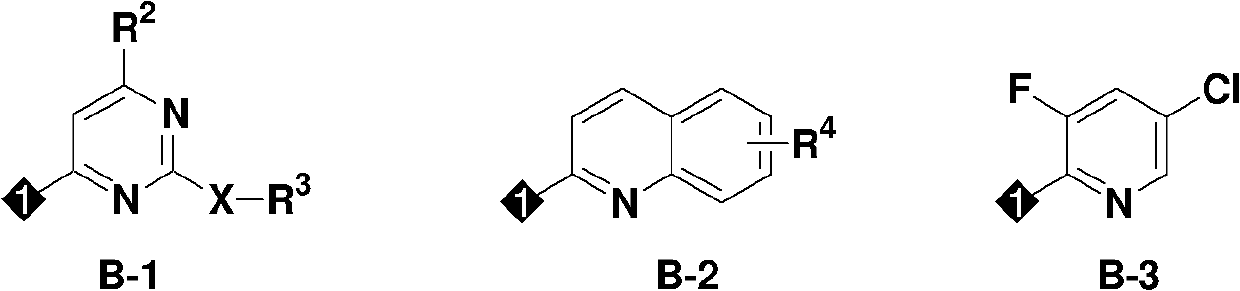
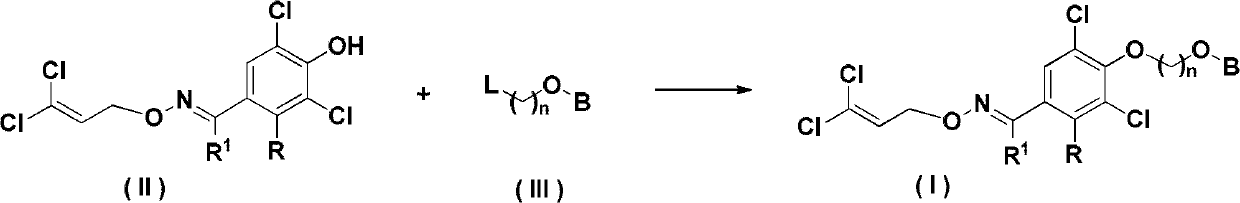
Insecticidal Oxime Ether Dichloroallyl Ether Compounds
A technology of dichloroallyl ether and insecticidal activity, applied in the direction of biocides, chemicals for biological control, biocides, etc., to achieve the effect of preventing insects and/or mites from infesting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074]
[0075] Step A: Synthesis of 2-methoxy-4-hydroxy-6-trifluoromethylpyrimidine (01-A)
[0076] Add 1.00 g of cyanamide into a 100 ml three-necked flask with a drying condenser, and add 5.00 g of HCl in methanol while stirring. Slowly raise the temperature to 25-80°C and react for 2-6 hours, cool in an ice-water bath to about 10°C, then add 3.50 g of ethyl trifluoroacetoacetate dropwise, and adjust the pH value to 14 with 20% NaOH solution after the dropwise addition. Control the temperature below 30°C, raise the temperature to reflux after the addition, react for 5 to 6 hours, and desolvate under reduced pressure, add 20 grams of water to adjust the pH to be acidic, until the amount of precipitated solids is the largest, and dry to obtain about 3.0 g of the crude product. Content 96% (HPLC).
[0077] Step B: Synthesis of 2-methoxy-4-chloro-6-trifluoromethylpyrimidine (01-B)
[0078] Add 3.88g (20mmol) 2-methoxy-4-hydroxy-6-trifluoromethylpyrimidine and 9.20g (60mmol...
Embodiment 2
[0088] Preparation of suspending agent: Dilute 2-6% wetting and dispersing agent in 4-10% antifreeze, and slowly add a certain amount of water to the solution, then add in order under high-speed shearing knife stirring 5-80% active compound of formula (I) provided by the present invention, 0.01-0.05% preservative, 0.01-0.05% defoamer and thickener, etc. Finally, it is poured into a sand mill for grinding, and then the solvent is added to volume. It can be diluted with water to any desired concentration when used.
Embodiment 3
[0090] First dilute 5 parts of suitable surfactants such as sodium naphthalenesulfonate formaldehyde condensate and dibutylnaphthalenesulfonate sodium formaldehyde condensate in 5 parts of suitable antifreeze such as ethylene glycol and glycerol, and add to the solution Slowly add water, add 10 parts of active compounds provided by the invention and other suitable additives such as preservatives (benzoic acid or formaldehyde, etc.), defoamers (organosilicone) and thickeners (yellow) under rapid stirring. Original acid gum or carboxymethyl cellulose, etc.), grind it after adding, and finally add the remaining solvent to volume.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com