Diaphragm for electrochemical synthesis of dinitrogen pentoxide and preparation method thereof
A dinitrogen pentoxide and electrochemical technology, applied in the direction of organic diaphragms, etc., can solve problems such as constraints, current efficiency and theoretical value gap, and achieve the effects of reducing losses, reducing diaphragm resistance, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
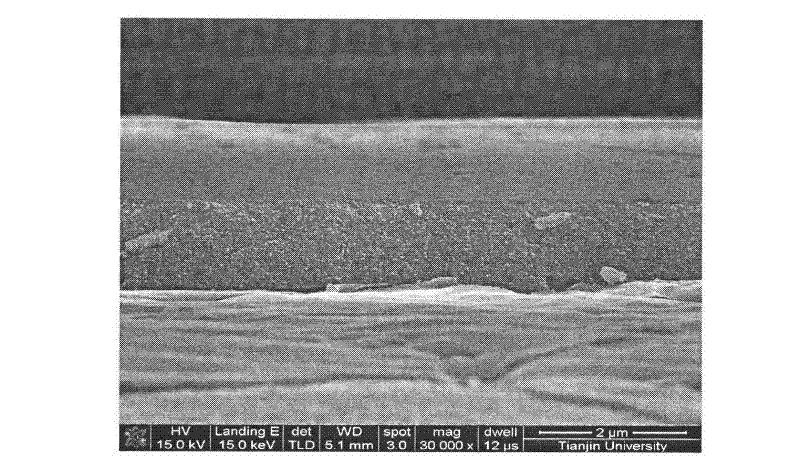
[0024] 1. The porous polytetrafluoroethylene membrane adopts the purchased BS01 hydrophobic porous polytetrafluoroethylene membrane. The size of the membrane is 5cm×5cm, the pore diameter is 0.1μm, the porosity is 55%, and the thickness is 0.06mm. The base film was refluxed in acetone at a temperature of 60°C for 3 hours, then immersed in 98% nitric acid for 24 hours, rinsed with deionized water after taking it out until the pH value of the eluate was 7, and dried in vacuum at a temperature of 80°C 24h. The dry film mass was determined to be 0.5397 g by weighing.
[0025] 2. Immerse the treated BS01 hydrophobic porous polytetrafluoroethylene membrane in a 5wt% high fluorinated ion exchange resin (Nafion) solution for 24h, dry it in a vacuum oven at 80°C for 20h, and weigh it to determine that the dry film quality is 0.5580 g, swell with deionized water for 24h, and then use 0.5mol / L H 2 SO 4 The solution was swollen for 4 hours to obtain a porous polytetrafluoroethylene-hig...
Embodiment 2
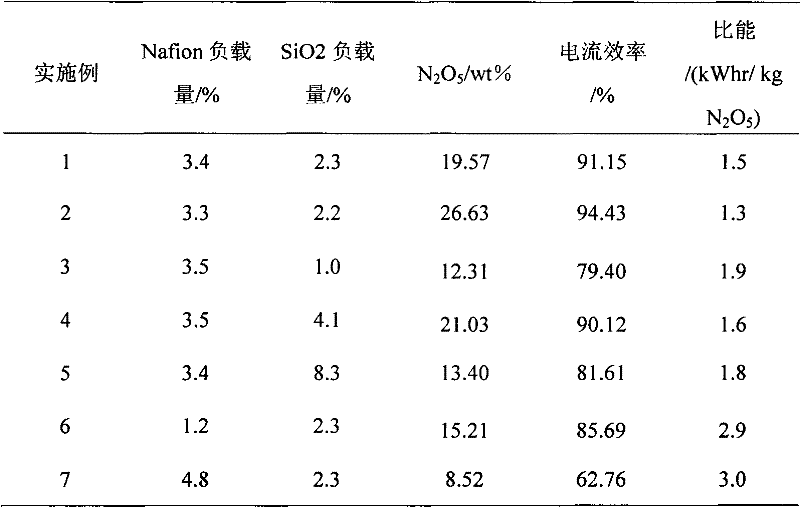
[0030] Steps 1, 2, and 3 are the same as in Example 1: a highly fluorinated ion-exchange resin (Nafion) with a loading capacity of 3.3%, SiO 2 Porous polytetrafluoroethylene-highly fluorinated ion exchange resin (Nafion)-SiO with a loading of 2.2% 2 membrane;
[0031] Step 4 is different from Example 1: the expanded polytetrafluoroethylene membrane is changed to ePTFE-2 type, with a pore size of 90-100 nm, a thickness of 10-20 μm, a porosity of 90-98%, and a mechanical pressing pressure of 10 MPa. The time is 50h, and the prepared membrane is porous polytetrafluoroethylene-high fluorinated ion exchange resin (Nafion)-SiO 2 - expanded polytetrafluoroethylene (ePTFE) diaphragm;
[0032] Step 5 is different from Example 1: the anode initial solution is N 2 o 4 Concentration of 28.1% N 2 o 4 / HNO 3 , the operating voltage is 5.5V.
[0033] The experimental results are shown in Table 1.
Embodiment 3
[0035] Steps 1 and 2 are the same as in Example 1: a porous polytetrafluoroethylene-highly fluorinated ion exchange resin (Nafion) membrane with a loading capacity of 3.5% was prepared;
[0036] The difference between step 3 and embodiment 1: change the film made of above SiO 2 Soak in the sol for 12 hours, dry at room temperature, then vacuum dry in an oven at 60°C for 12 hours, weigh to determine the quality of the dry film, and obtain SiO 2 Porous polytetrafluoroethylene-highly fluorinated ion exchange resin (Nafion)-SiO with a loading of 1.0% 2 membrane;
[0037] Step 4 is the same as Example 1: the membrane obtained is porous polytetrafluoroethylene-high fluorinated ion exchange resin (Nafion)-SiO 2 - expanded polytetrafluoroethylene (ePTFE) diaphragm;
[0038] Step 5 is different from Example 1: the anode initial liquid is N 2 o 4 Concentration of 30.1% N 2 o 4 / HNO 3 , operating voltage is 4.5V.
[0039] The experimental results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com