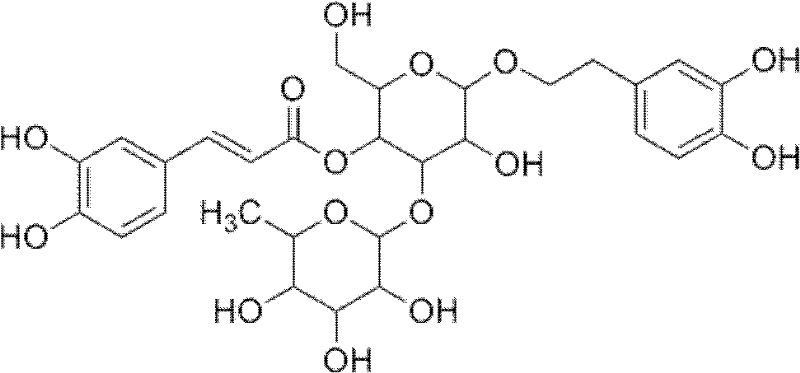
Application of ergot sterioside
An ergosteroid, a single technology, applied in the field of traditional Chinese medicine, can solve problems such as side effects, liver and kidney toxicity, and adverse reactions, and achieve the effects of promoting glucose consumption, low cytotoxicity, and reducing fasting blood sugar levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 1 kg of Semen psyllium coarse powder, extract with 4 times the volume of 70% ethanol aqueous solution under reflux for 3 hours, repeat 3 times. The combined extracts were filtered and concentrated to dry solvent.
[0020] The aqueous suspension of the above-mentioned concentrated extract obtained by diluting with water at a weight / volume ratio of 1:3 was extracted with petroleum ether (60-90°C) for several times until the petroleum ether layer was nearly colorless, and the petroleum ether layer was discarded ; The aqueous layer was extracted with saturated n-butanol until the n-butanol layer was nearly colorless, and the n-butanol layers were combined and concentrated under reduced pressure.
[0021] Through silica gel column chromatography, gradient elution was carried out with an ethyl acetate-methanol-water solution system with a volume ratio of 35:1:0.4, and the eluate was collected and concentrated under reduced pressure to obtain crude ergosteroside.
[0022...
Embodiment 2
[0026] HepG2 cells were inoculated in a 96-well plate, and when the cells grew to 60% confluent, they were replaced with a culture medium containing 0.2% bovine serum albumin (BSA) of different concentrations of ergosteroside, and after incubation for 24 hours, the glucose in the culture medium was detected. For the remaining amount, calculate the glucose consumption rate of cells in each well for 24 hours according to the following formula.
[0027] Glucose consumption rate = (the measured value of the experimental well at 0h-the measured value of the experimental well at 24h) / (the measured value of the control well at 0h-the measured value of the control well at 24h)×100%.
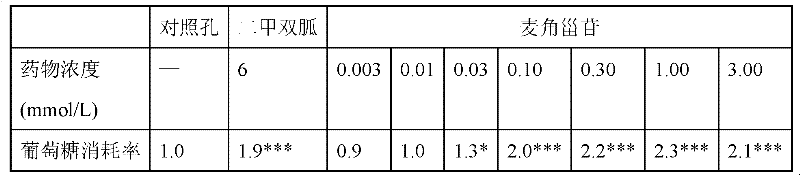
[0028] Table 1 Promoting effect of ergosteroid on glucose metabolism in HepG2 cells
[0029]
[0030] Note: Metformin is a positive drug. * P*** P<0.001, compared to control wells.
[0031] It can be seen from Table 1 that ergosteroid can significantly promote the glucose metabolism of HepG2 cells. ...
Embodiment 3
[0033] At the end of the HepG2 cell glucose consumption test, remove the culture medium to be tested, replace it with a culture medium containing 0.5g / LMTT and continue to incubate for 4 hours, then pour out the culture medium, blot dry, add 200 μl dimethyl sulfoxide to each well, and mix. After uniformity, put it on a microplate reader to measure the absorbance at 570nm, and calculate the survival rate of HepG2 cells according to the following formula: cell survival rate=measured value of experimental wells / measured value of control wells×100%.
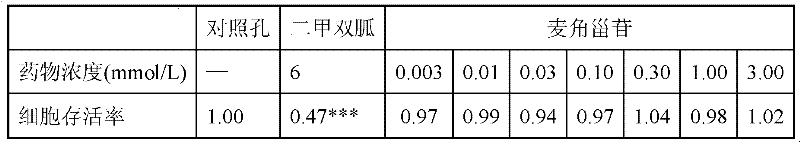
[0034] Table 2 Toxicity of ergosteroids to HepG2 cells
[0035]
[0036] Note: Metformin is a positive drug. *** P<0.001, compared to control wells.
[0037] It can be seen from Table 2 that compared with the positive control drug metformin, ergosteroside has almost no toxicity to HepG2 cells, showing very good safety.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com