Method for refining pharmaceutical dibenzothiazyl disulfide
A technology of dibenzothiazole disulfide and a refining method, applied in the direction of organic chemistry, etc., can solve the problems of increasing the allergy of the final drug, high consumption of toluene, harm to operators, etc., and achieve excellent physical and chemical properties and reactivity, and solvent cost. Low, low overall cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
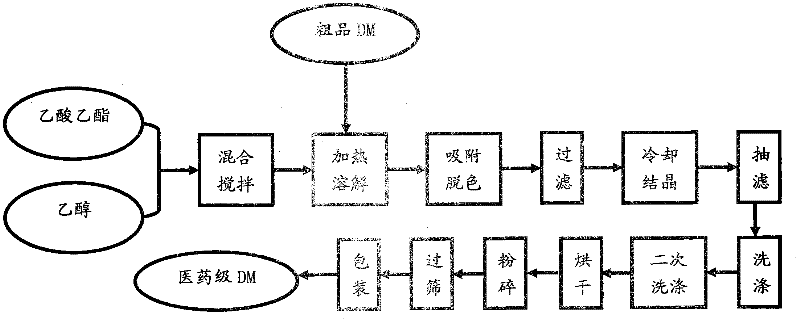
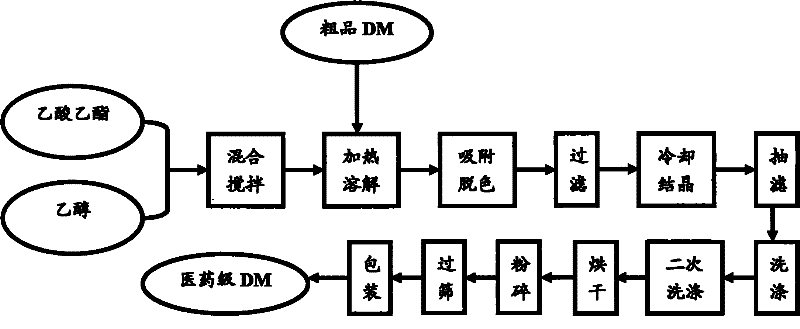
[0024] The raw materials used in the present invention are crude product DM, solvent ethyl acetate, solvent ethanol;
[0025] Raw material ratio:
[0026] Crude product DM (g): solvent ethyl acetate (g) = 1.00: 0.37
[0027] Solvent ethyl acetate (ml): solvent ethanol (ml) = 1.0: 7.5
[0028] Put 100ml of solvent ethyl acetate into 760ml of ethanol, under stirring, put in 243g of crude product DM, control the temperature at 65°C to dissolve it completely, add 0.1% activated carbon to the solution, stir for 1 hour, and filter with suction to separate the activated carbon. The mother liquor was cooled to 25°C, and DM crystals were precipitated. Stop stirring, filter with suction, and wash with ethyl acetate: ethanol mixture with a volume ratio of 1.0:7.5 to obtain refined wet product DM, which is dried, pulverized, sieved, and packaged to obtain pharmaceutical grade DM. It is determined that the reaction yield is 96.2%, the initial melting point of the product is 180.3°C, the...
Embodiment 2
[0030] The raw material used in the present invention is crude product DM, solvent ethyl acetate, solvent ethanol;
[0031] Raw material ratio:
[0032] Crude product DM (g): solvent ethyl acetate (g) = 1.00: 0.39
[0033] Solvent ethyl acetate (ml): solvent ethanol (ml) = 1.0: 6.9
[0034] Put 100ml of solvent ethyl acetate into 690ml of ethanol, put in 231g of crude product DM under stirring, control the temperature at 70°C to dissolve it completely, add 1% activated carbon to the solution, stir for 1.5h, and filter with suction to separate the activated carbon , the mother liquor was cooled to 20°C, and DM crystals were precipitated. Stop stirring, filter with suction, and wash with ethyl acetate: ethanol mixture with a volume ratio of 1.0:6.9 to obtain refined DM as a wet product. Dry, pulverize, sieve, and pack to obtain pharmaceutical grade DM. The reaction yield is 96.7%, the initial melting point of the product is 180.6°C, the purity is 99.2%, and the appearance is ...
Embodiment 3
[0036] The raw material used in the present invention is crude product DM, solvent ethyl acetate, solvent ethanol;
[0037] Raw material ratio:
[0038] Crude product DM (g): solvent ethyl acetate (g) = 1.00: 0.45
[0039] Solvent ethyl acetate (ml): solvent ethanol (ml) = 1.0: 6.5
[0040] Put 100ml of solvent ethyl acetate into 620ml of ethanol, under stirring, put in 200g of crude product DM, control the temperature at 72°C to dissolve it completely, add 2% activated carbon to the solution, stir for 2 hours, and filter with suction to separate the activated carbon. The mother liquor was cooled to 15°C, and DM crystals were precipitated. Stop stirring, filter with suction, and wash with ethyl acetate: ethanol mixture with a volume ratio of 1.0:6.5 to obtain refined DM as a wet product. Dry, pulverize, sieve, and pack to obtain pharmaceutical grade DM. The reaction yield is 97.5%, the initial melting point of the product is 181.4°C, the purity is 99.2%, and the appearance ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com