Roxithromycin monohydrate crystal, its preparation method and composition dry suspension containing the combination of the crystal and ambroxol hydrochloride
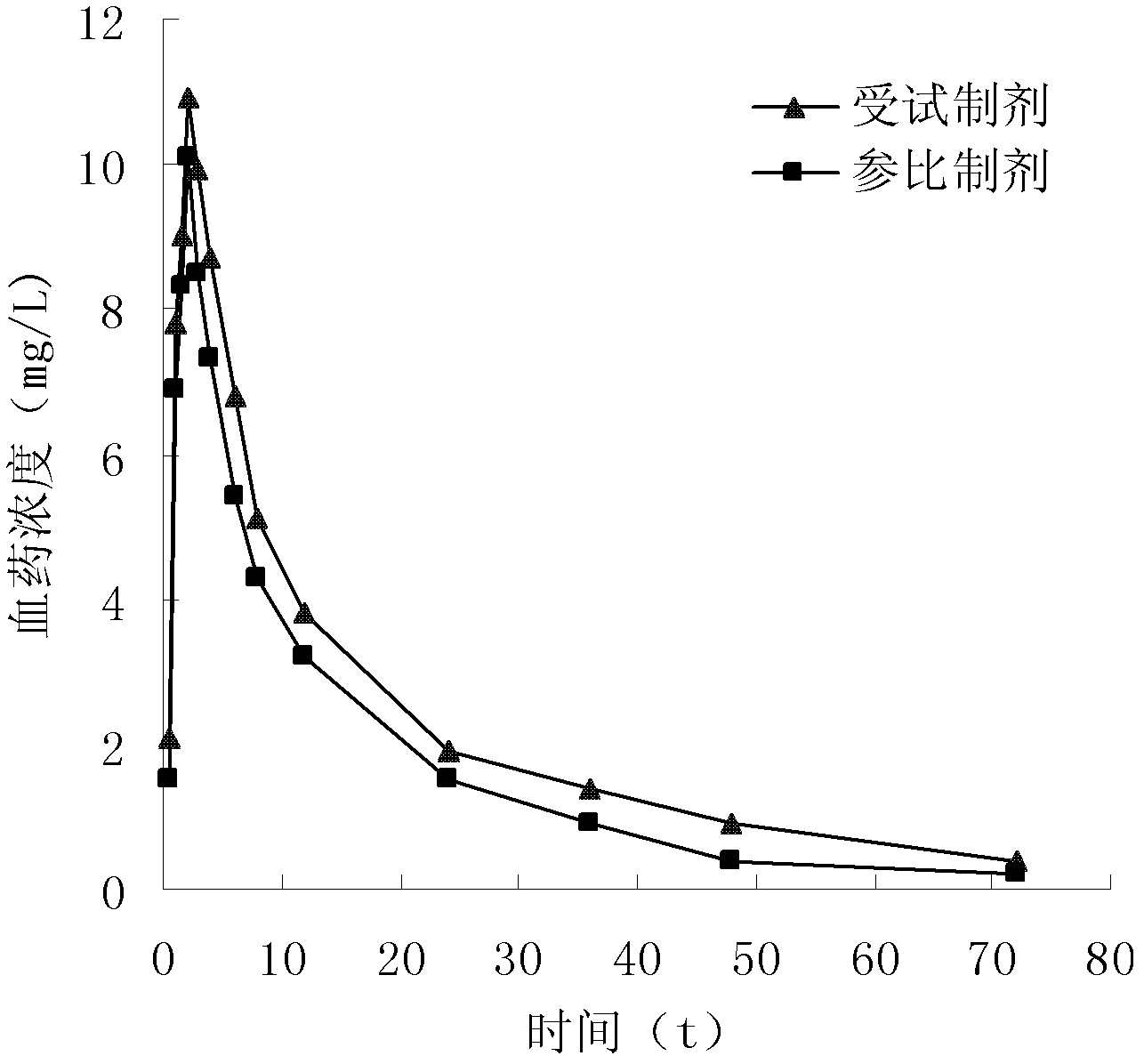
A technology of roxithromycin and monohydrate, applied in the field of pharmaceutical preparations, can solve the problems of reduced drug bioavailability, inability to obtain antibacterial effect, and difficulty in achieving complete dissolution, and achieves improved bioavailability, improved drug dissolution, The effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
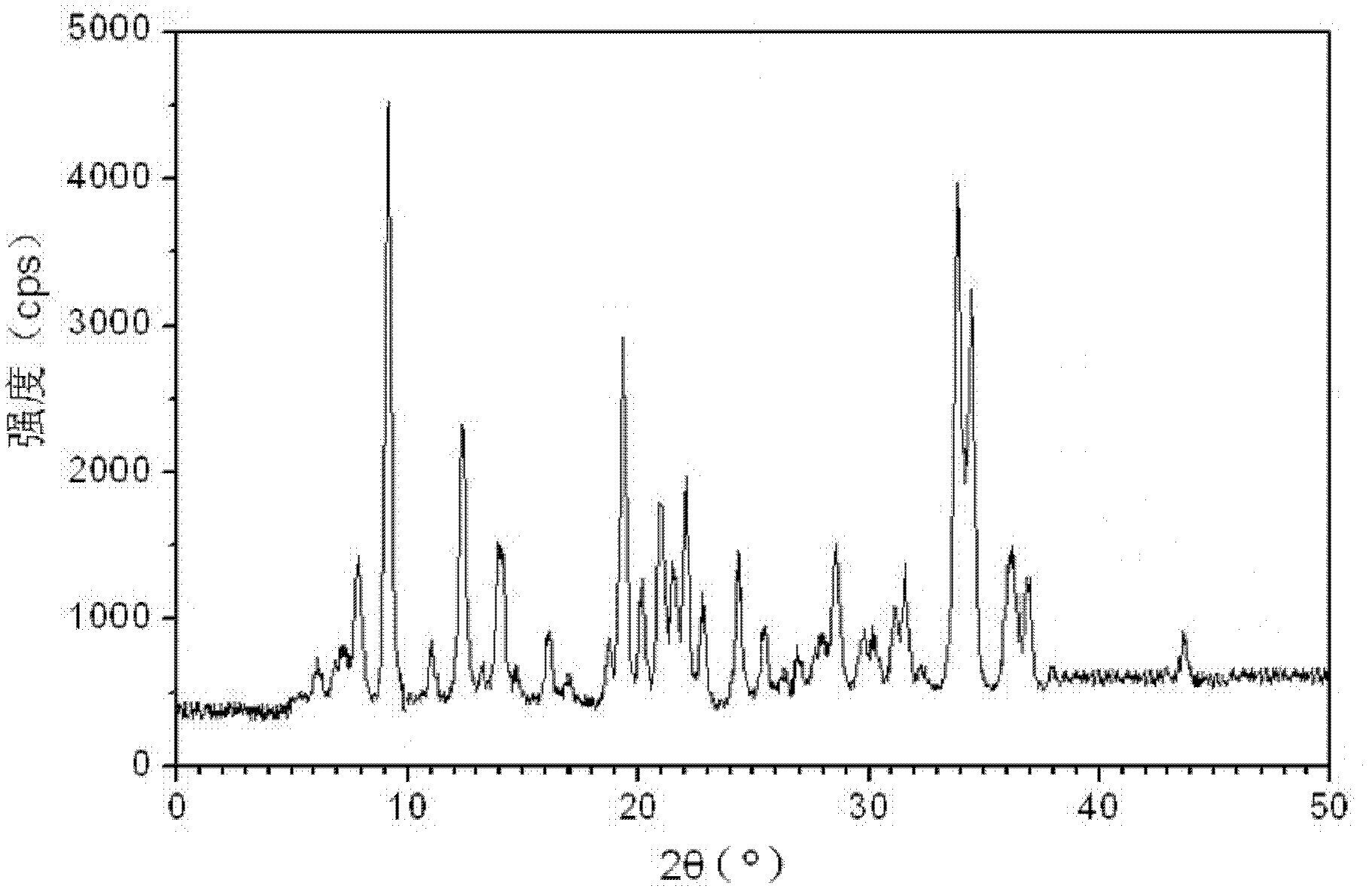
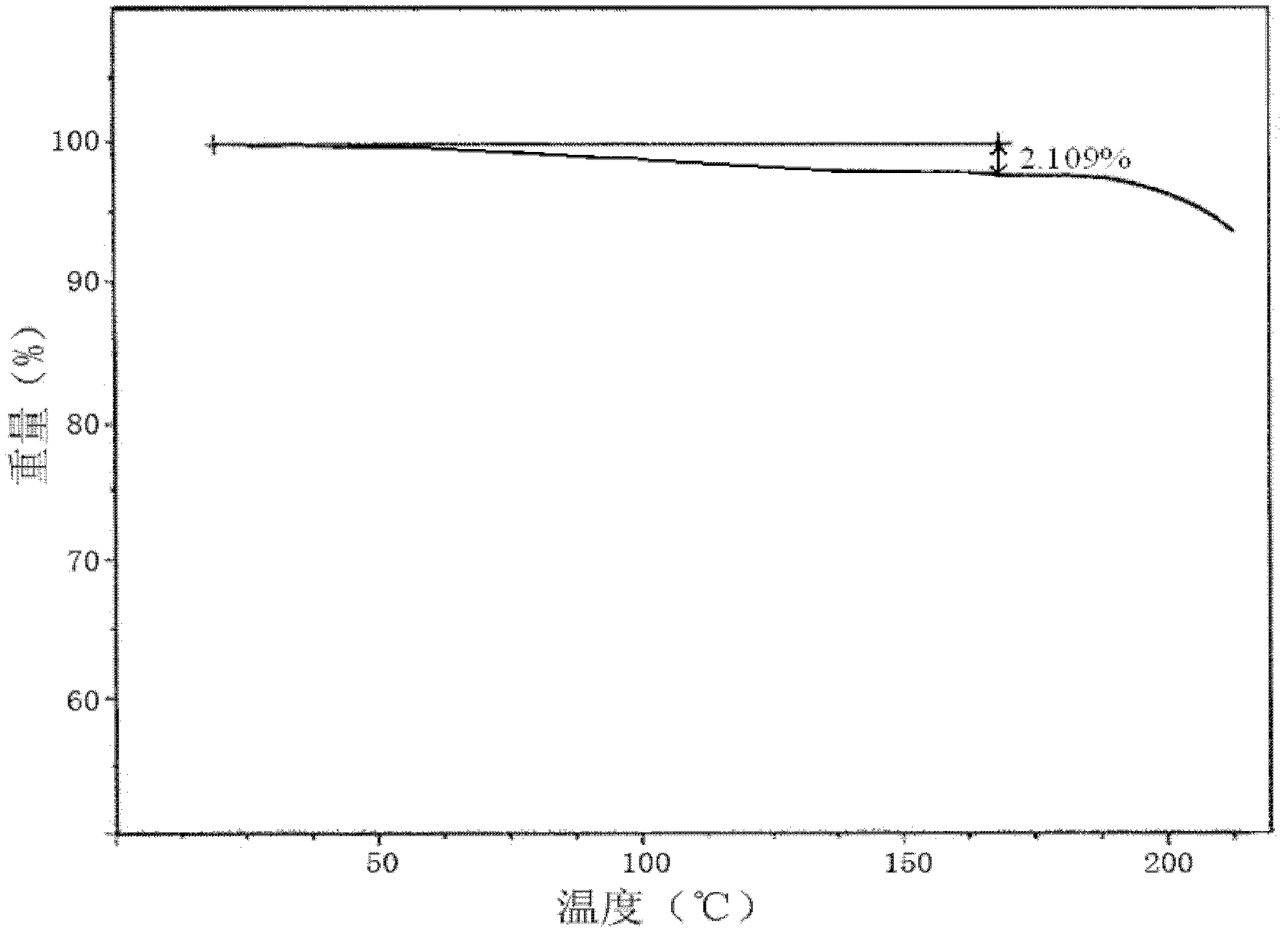
[0063] [embodiment 1] the preparation of roxithromycin monohydrate crystal
[0064] 1) Sample dissolution: under the condition of 40-50°C, dissolve 1 kg of roxithromycin in a mixed solution of 5 kg of anhydrous methanol and 1 kg of acetone to obtain solution I, and keep the temperature of solution I at 38-42° C., wherein;
[0065] 2) Crystallization: Take solution I and press filter to remove insoluble matter to obtain solution II. Keep the temperature of solution II at 38-42°C. Under stirring, continuously add 1 / 4-2 / 5 of the volume of solution II pure water dropwise, and control it at 1 Add within ~2 hours; after crystal growth for 1~2 hours, continue to drop 3 / 5~3 / 4 of the volume of pure water of solution II under stirring, control the addition within 1~2 hours, and stir until crystallization ; After growing crystals for 1-2 hours, cool down to 15-18°C, keep warm for 1-3 hours, centrifuge, and collect the wet product of roxithromycin;
[0066] 3) Pulverization: pulverize th...
preparation Embodiment 1
[0075] [Preparation Example 1] Preparation of Roxithromycin Ambroxol Composition Dry Suspension
[0076] Prescription:
[0077]
[0078]
[0079] Preparation Process:
[0080] 1) The raw and auxiliary materials ambroxol hydrochloride, sucrose, xanthan gum and hypromellose are pulverized through an 80-mesh sieve for subsequent use;
[0081] 2) Take the roxithromycin monohydrate crystals prepared in Example 1 according to the stated amount, mix them with the crushed and sieved raw and auxiliary materials in step 1), add an appropriate amount of banana essence, mix well, and pack it have to.
preparation Embodiment 2
[0082] [Preparation Example 2] Preparation of Roxithromycin Ambroxol Composition Dry Suspension
[0083] Prescription:
[0084]
[0085] Preparation process: same as formulation example 1, the difference is that the roxithromycin monohydrate crystals used are the roxithromycin monohydrate crystals prepared in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com