Scale-scale purification of whey protein
A purification method and technology of whey protein, applied in the direction of albumin peptide, animal/human protein, animal/human peptide, etc., can solve the problems of low selectivity, low efficiency, many operation steps, etc., to reduce production cost, low cost, etc. cost, the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
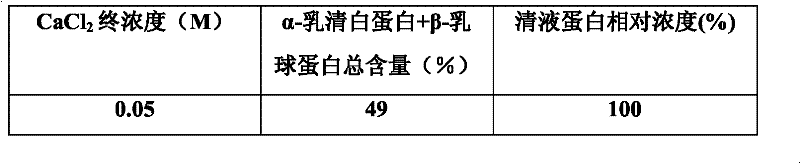
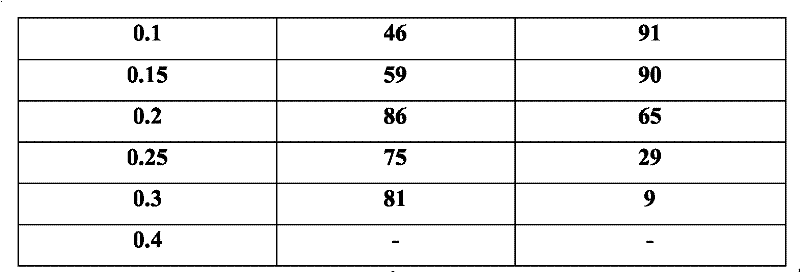
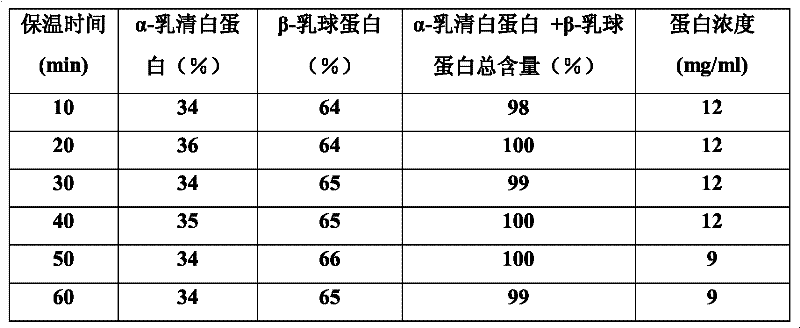
[0013] Take the reconstituted milk powder, add deionized water at a ratio of 1:8 (w / v), centrifuge and degrease, take the whey and divide it into multiple parts, and adjust the CaCl 2 The final concentration is 0.0, 0.05, 0.1, 0.15, 0.2, 0.25, 0.3, 0.4M, and then heated to the temperature: 70°C, 75°C, 80°C, 85°C, 90°C, 95°C for 10min, 20min, 30min, 40min, 50min, 60min; after the reaction is completed, centrifuge each tube at 4°C, 9800rpm for 10min, transfer the supernatant to a new tube and add NaCO 3 , to remove CaCl by centrifugation 2 , with 200mL pH 7.0, 10mM sodium phosphate buffer solution to wash the precipitate three times and dissolve it. 13.5% SDS-PAGE was used to analyze the composition of supernatant and precipitated protein, and the supernatant was determined for protein quantification. The total content of total α-lactalbumin and β-lactoglobulin in the concentrated sample can reach more than 99%. Table 1 shows whey samples at different concentrations of CaCl ...
Embodiment 2
[0024] The whey pretreatment sample prepared in Example 1 was adjusted to pH 7.4 with 1M HCl or NaOH solution, and loaded onto a Q Sepharose Fast Flow anion-exchange chromatography column (11x22cm) pre-equilibrated with 50mM, pH 7.4 Tris-HCl buffer , flow rate 200ml / ml; after sample loading, wash the column with 50mM, pH 7.4 Tris-HCl buffer until the absorption signal at 280nm reaches the baseline, and elute with NaCl-50mM Tris-HCl (pH 7.4) buffer with a conductivity of 10.2ms / cm . 1.5L of recombinant human α-lactalbumin fraction was collected with a recovery rate of 76%; the purity of recombinant human α-lactalbumin detected by high performance liquid chromatography was 94.1%; 1.2L of bovine α-lactalbumin peak was collected and recovered by the process The yield is 79.2%, and the purity of bovine α-lactalbumin detected by high performance liquid chromatography is 90.1%; the bovine β-lactoglobulin peak 2L is collected, and the process recovery rate is 85.5%, and the purity of ...
Embodiment 3
[0026] The whey pretreatment sample prepared in Example 1 was adjusted to pH 6.4 with 1M HCl or NaOH solution, and loaded onto a Q Sepharose Fast Flow anion-exchange chromatography column (11x22cm) pre-equilibrated with 50mM, pH6.4 Tris-HCl buffer , the flow rate is 200ml / ml; after loading the sample, wash the column with 50mM, pH6.4 Tris-HCl buffer until the absorption signal at 280nm reaches the baseline, and then wash with NaCl-50mM Tris-HCl (pH6.4) buffer with a conductance of 10.2ms / cm take off. 1.6L of recombinant human α-lactalbumin fraction was collected with a recovery rate of 72%; the purity of recombinant human α-lactalbumin detected by HPLC was 91.2%; bovine α-lactalbumin peak 1.3L was collected with a recovery rate of 75.3%, the purity of bovine α-lactalbumin detected by HPLC is 87.3%; the bovine β-lactoglobulin peak 2.1L is collected, the recovery rate is 90.7%, the purity of bovine β-lactoglobulin detected by HPLC is 75.8% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com