Kits and methods for direct nucleic acid amplification from whole blood samples
A whole blood sample and nucleic acid technology, applied in the field of nucleic acid amplification, can solve problems such as cumbersome operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~27
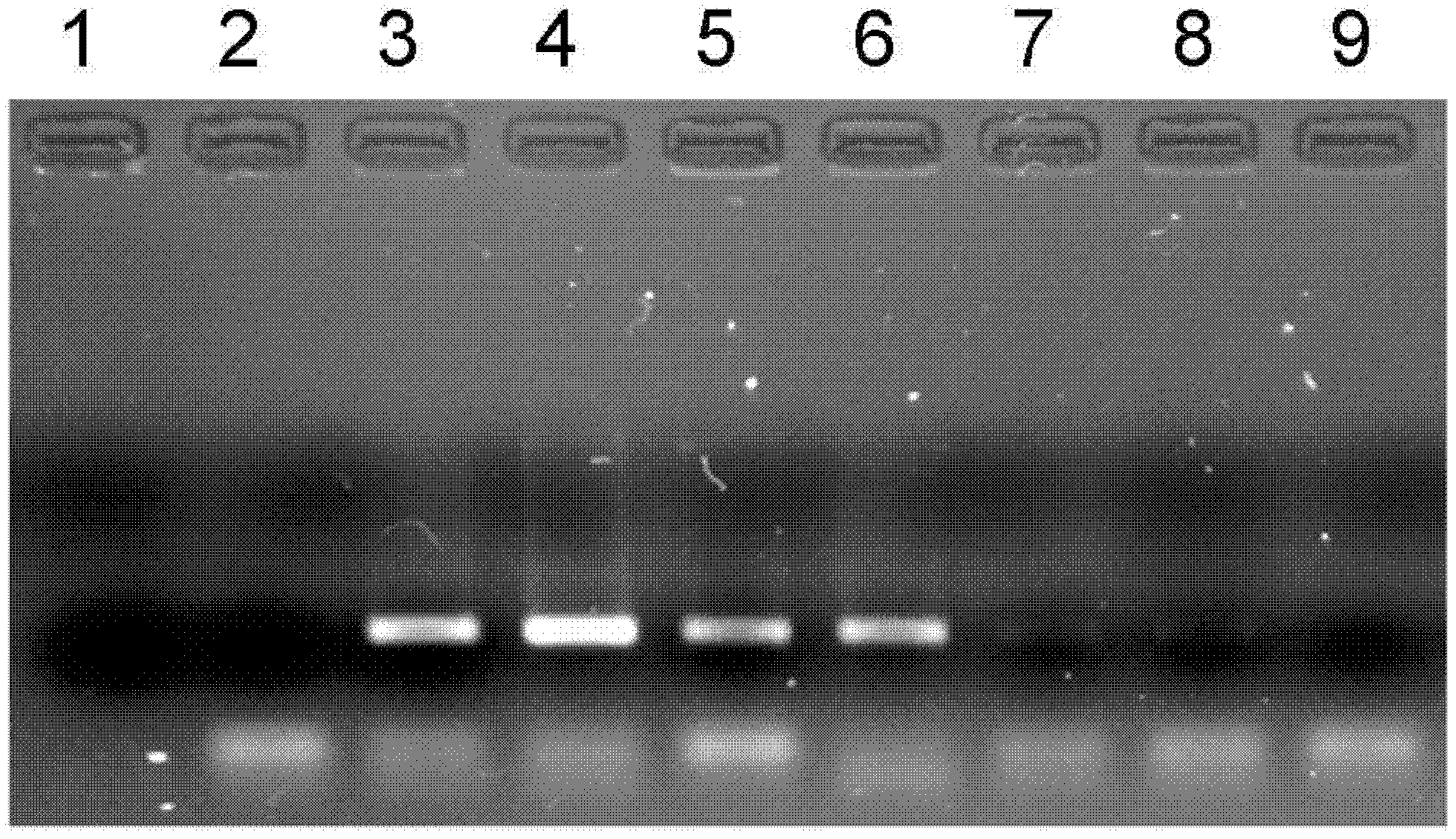
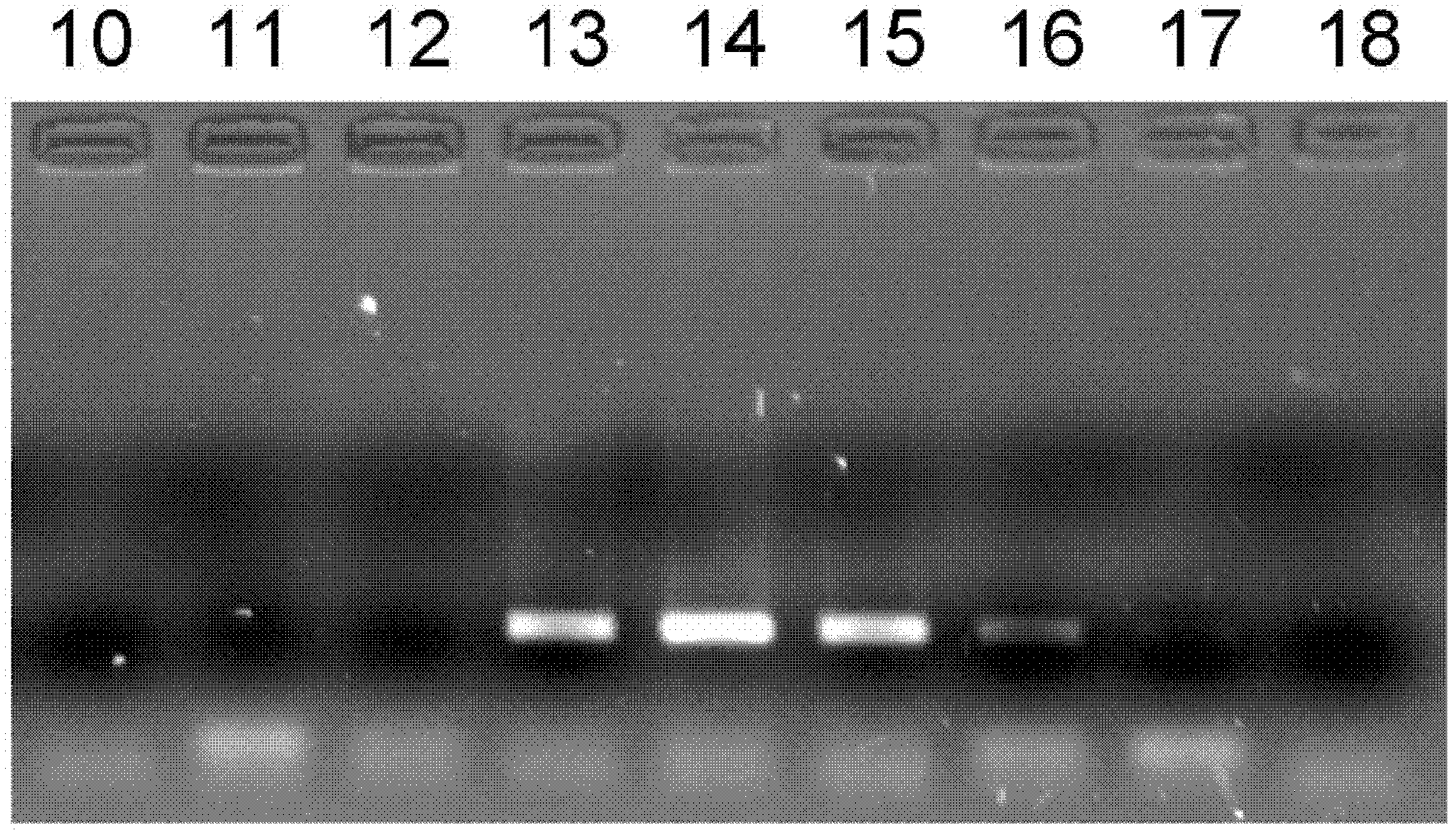
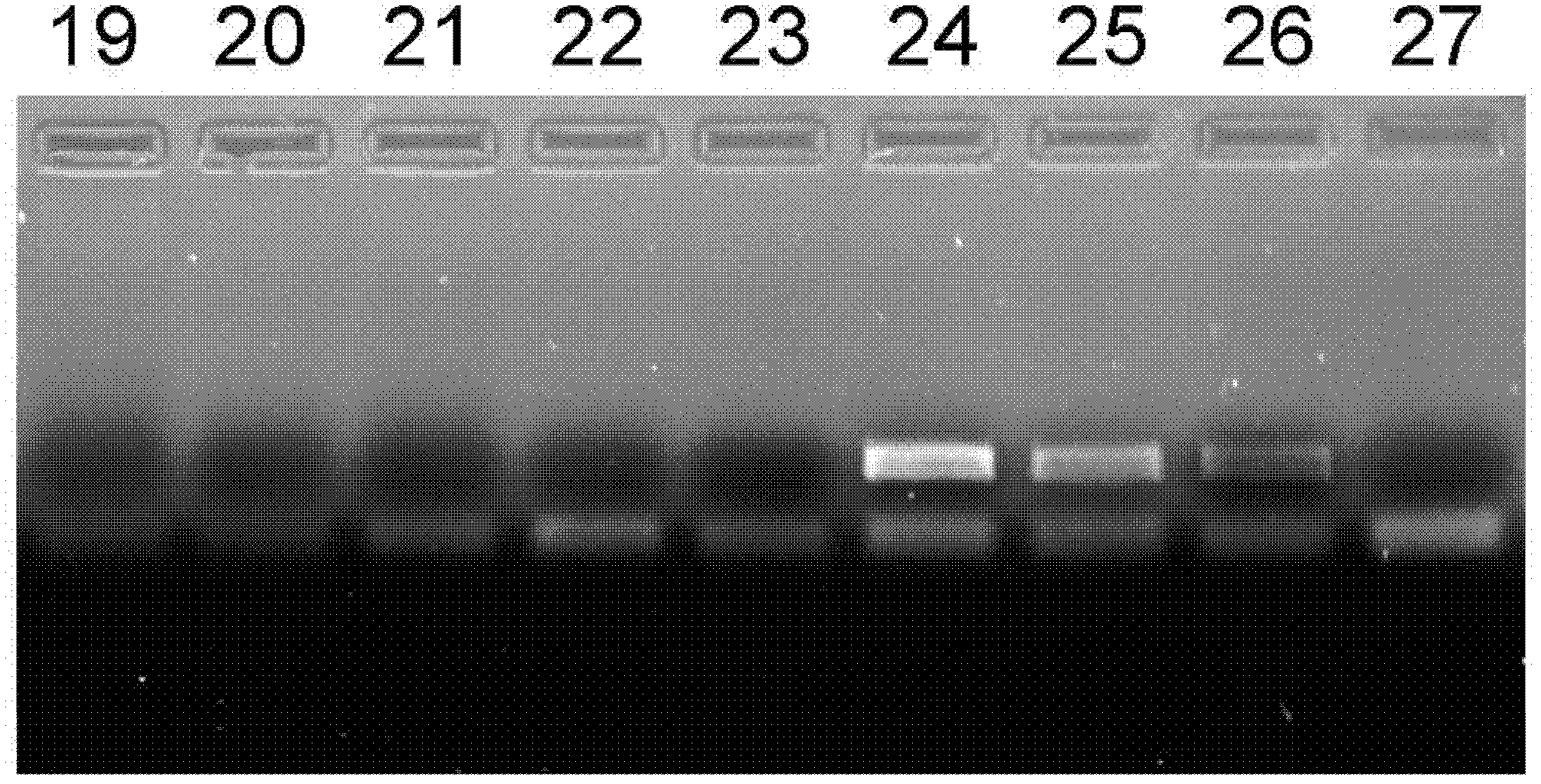
[0054] The optimization experiment of embodiment 1~27 PCR amplification reaction system
[0055] This embodiment uses a single factor optimization test to detect the impact of PCR amplification reaction solutions containing different concentrations of tris, magnesium ions, and glycerol on the amplification efficiency; wherein in Examples 1 to 9, the concentrations of Tris bases are respectively controlled at 0, 10, 25, 50, 75, 100, 125, 150 and 200mmol / L, magnesium ion concentration adopts 2.0mmol / L, glycerin concentration is 10%, other conditions are stable; embodiment 10~18 controls magnesium ion concentration respectively At 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0mmol / L; Tris alkali concentration is controlled at 50mmol / L respectively, and glycerol concentration is 10%, and other conditions are stable; Embodiment 19~27 makes glycerol concentration Respectively controlled at 0, 2%, 4%, 6%, 8%, 10%, 12%, 14% and 16%, Tris base concentration was controlled at 50mmol / L, ma...
Embodiment 28
[0065] Example 28 Detection of single nucleotide polymorphisms associated with esophageal cancer
[0066] This example is used to simultaneously determine three single nucleotide polymorphism sites associated with esophageal cancer
[0067] 1. Experimental materials: blood samples treated with different anticoagulants (ethylenediaminetetraacetic acid disodium salt, sodium citrate); genomic DNA, extracted by phenol / chloroform extraction; 9 primer sequences are shown in Table 1, Synthesized by Jinsite Technology (Nanjing) Co., Ltd., PAGE grade; other materials are the same as in Example 1.
[0068] Genomic DNA was extracted using the following methods:
[0069] 1) Sampling: Take 1-2ml of peripheral blood from the patient and place it in sodium citrate (1:9) or EDTA anticoagulant tubes instead of heparin anticoagulant tubes. Aspirate part of the blood in the anticoagulant tube and centrifuge at 8000rpm for 5 minutes to separate plasma and cells. Carefully transfer the plasma i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com