Novel Fibroblast Growth Factor Receptor Tyrosine Kinase Inhibitors
A technology of growth factor receptors and fibroblasts, applied in the field of medicine, can solve the problems of drug resistance, reduce drug targeting selectivity, increase in vivo toxicity, etc., to overcome drug resistance, inhibition, microvascular generate inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] General Synthesis of Compounds
[0031] Synthesis of compounds L50H1, L50H4, L50H25, L50H34, L60H34 and L61H27:
[0032] Dissolve 2mmol of the corresponding substituted benzaldehyde in 10mL of absolute ethanol, add 1mmol each of the corresponding piperidin-4-one, tetrahydrothiopyran-4-one and tetrahydropyran-4-one, 5-8°C Slowly add 5-10 drops of sodium methoxide solution under low temperature, and react at 5-8°C for 5-24h, then use TLC to detect the progress of the reaction. After the reaction, add 1-2 times the volume of the reaction solution in water to precipitate a yellow precipitate, filter it with suction, and dry it under vacuum at 30°C overnight to obtain a yellow powder product, which is purified by silica gel column chromatography to obtain compounds with a purity greater than 98%.
[0033] Synthesis of L50H23, L49H23, L60H23 and L61H23:
[0034] Dissolve 2mmol of the corresponding 3,4-dihydroxybenzaldehyde in 10mL of absolute ethanol, add the corresponding ...
Embodiment 2
[0058] Inhibitory activity of compounds against FGFR1 and FGFR3 tyrosine kinases
[0059] FGFR1 tyrosine kinase inhibitory activity experiments were carried out on all the synthesized compounds. The experimental method is Caliper Mobility Shift Assay, which uses a detection system based on the mobility detection technology of microfluidic chip technology. The experimental steps are: configure 1.25x kinase reaction buffer (62.5mM HEPES, pH 7.5; 0.001875% Brij-35; 12.5mM MgCl 2 ; 2.5mM DTT) and kinase reaction stop solution (100mM HEPES, pH 7.5; 0.015% Brij-35; 0.2% Coating Reagent#3); in 5 μl of 5x concentration compound solution (dissolved in DMSO, diluted 10 times with water) Add 10 μl of 2.5x FGFR1 kinase solution (add kinase in 1.25x kinase reaction buffer), incubate at room temperature for 10 minutes, then add 10 μl of 2.5x substrate peptide solution (add FAM-labeled peptide in 1.25x kinase reaction buffer and ATP), add 25 μl kinase reaction stop solution after reacting a...
Embodiment 3
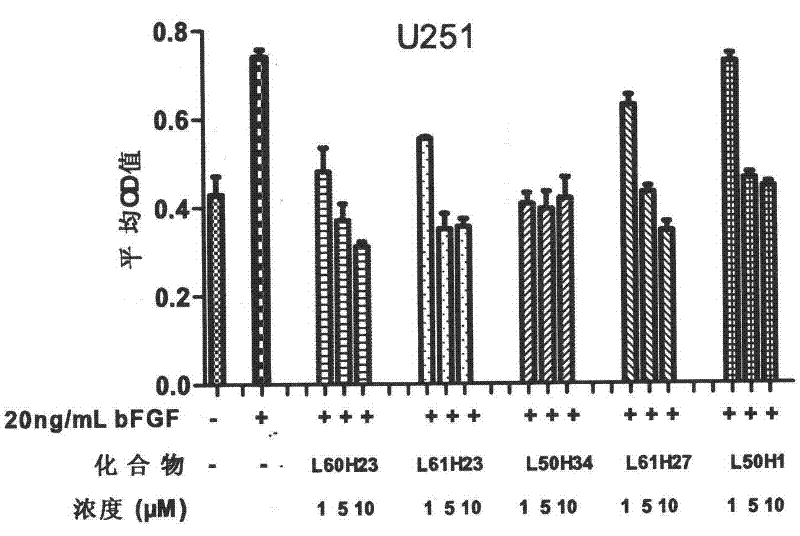
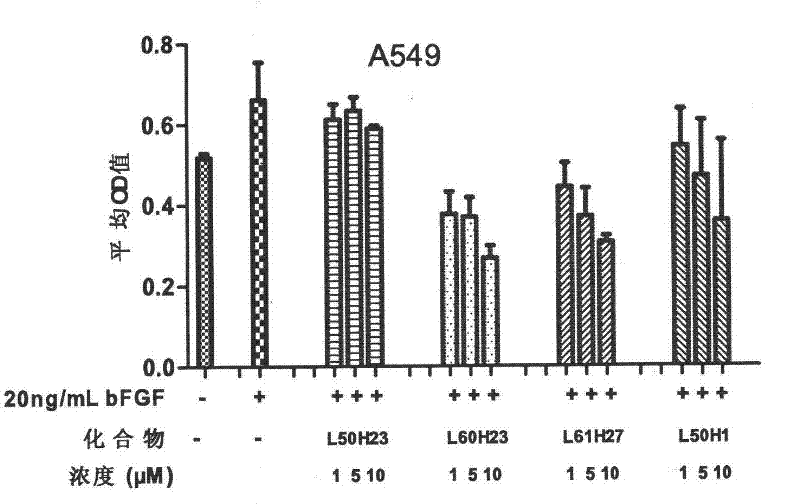
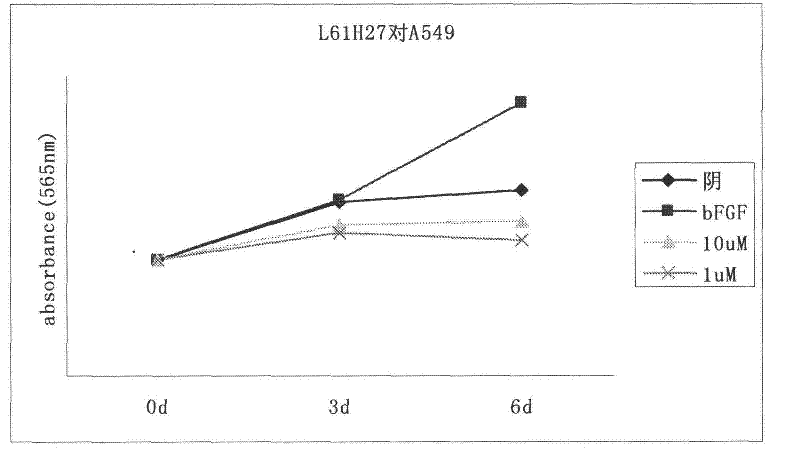
[0064] Proliferation inhibitory experiment of compounds on tumor cells with high expression of FGFR1
[0065] The cell proliferation inhibitory activity of the compound on FGFR1 high expression glioma cells (U251) and lung cancer cells (A549) was determined by MTT assay. The medium used for the cells is high-glucose DMEM medium with 10% (V / V) fetal bovine serum. Culture conditions are 37°C, 5% CO 2 After the logarithmic growing cancer cells were digested with trypsin solution, the corresponding culture medium was used to make cell suspension, added to a 96-well plate, 200 μL per well, 5000 cells / well, and cultured in an incubator for 24 hours. Then starve for 24h with the corresponding medium containing 0.4% fetal bovine serum. Aspirate the medium, and then add the corresponding medium containing 0.4% fetal bovine serum that has been added with the drug (concentration: 20 μg / mL). After incubation for 1 hour, add bFGF to make the final concentration 20 ng / mL, and continue to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com