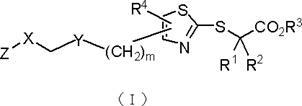
Carboxylic acid derivatives containing thiazole ring and their pharmaceutical use
A kind of technology of carboxylic acid derivatives and thiazole rings, applied in the field of new carboxylic acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
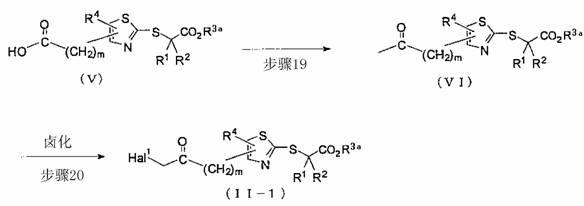
[0394] tert-butyl 2-{[4-(chloroacetyl)-1,3-thiazol-2-yl]thio}-2-methylpropionate
[0395]
[0396] Dissolve 2-(1-tert-butoxycarbonyl-1-methyl-ethylthio)-thiazole-4-carboxylic acid in dichloromethane (1200 mL), add 1-ethyl-3-(3-di methylaminopropyl)carbodiimide hydrochloride (191.70 g), 4-dimethylaminopyridine (5.86 g) and methanol (19.47 mL), and the mixture was stirred at room temperature overnight. The mixture was washed with dilute hydrochloric acid (about 0.5 mol / l), saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution (500 mL each), and dried over anhydrous sodium sulfate. The solvent was evaporated, and the residue was redissolved in ethyl acetate (700 mL), washed successively with dilute hydrochloric acid (about 0.2 mol / l), saturated aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution (300 mL each) , and then dried over anhydrous sodium sulfate. The solvent was evaporated, the resulting solid wa...
Embodiment 2
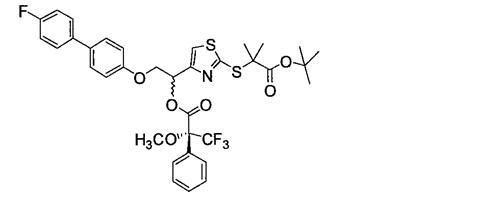
[0401] 2-[(4-{2-[(4'-fluorobiphenyl-4-yl)oxy]-1-hydroxyethyl}-1,3-thiazol-2-yl)thio]-2-methanol Propionic acid
Embodiment 2-1
[0403] 2-[(4-{[(4'-fluorobiphenyl-4-yl)oxy]acetyl}-1,3-thiazol-2-yl)thio]-2-methylpropanoic acid tert-butyl ester
[0404]
[0405] tert-butyl 2-{[4-(chloroacetyl)-1,3-thiazol-2-yl]thio}-2-methylpropanoate (23.8 g) obtained in Example 1 and 4'- Fluorobiphenyl-4-ol (12.00 g) was dissolved in toluene (650 mL), and aqueous sodium hydroxide solution (1 mol / l, 71 mL), tetrabutylammonium iodide (2.62 g) and water (71 mL), and the mixture was heated at reflux for 3 hours, allowed to cool, and partitioned. The organic layer was washed twice with 1 mol / L aqueous sodium hydroxide solution (300 mL), washed twice with saturated aqueous sodium chloride solution (200 mL), and dried over anhydrous sodium sulfate. The solvent was evaporated, and the obtained residue was purified by column chromatography (using Moritex Corporation Purif Pack SI 60 μm, specification 200. Elution solvent: hexane / ethyl acetate=95 / 151→70 / 30) to obtain as a pale yellow oily substance The title compound (1.128 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com