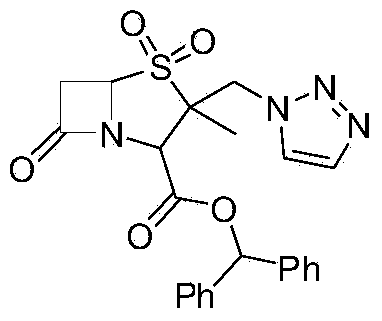
Method for preparing 2 beta-methyl penicillanate benzhydryl dioxide
A technology of triazolemethyl penicillanic acid diphenylmethyl ester and dioxide, which is applied in the field of preparation of 2β-triazolemethyl penicillanic acid diphenylmethyl ester dioxide, which can solve the limitation of large-scale production , Explosion in the synthesis process, complex synthesis technology and other problems, to achieve the effect of shortening the reaction cycle, low raw material cost and high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
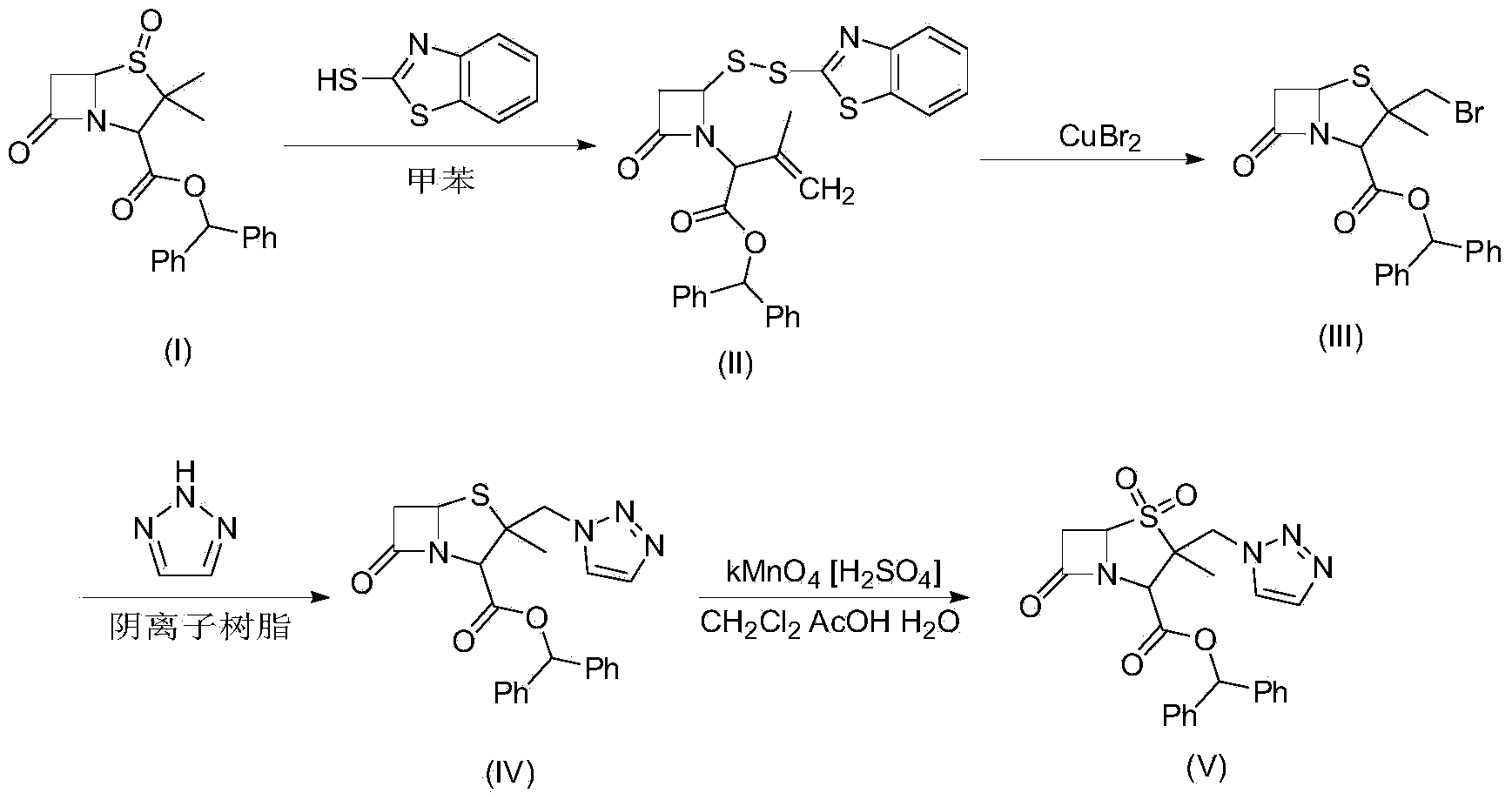
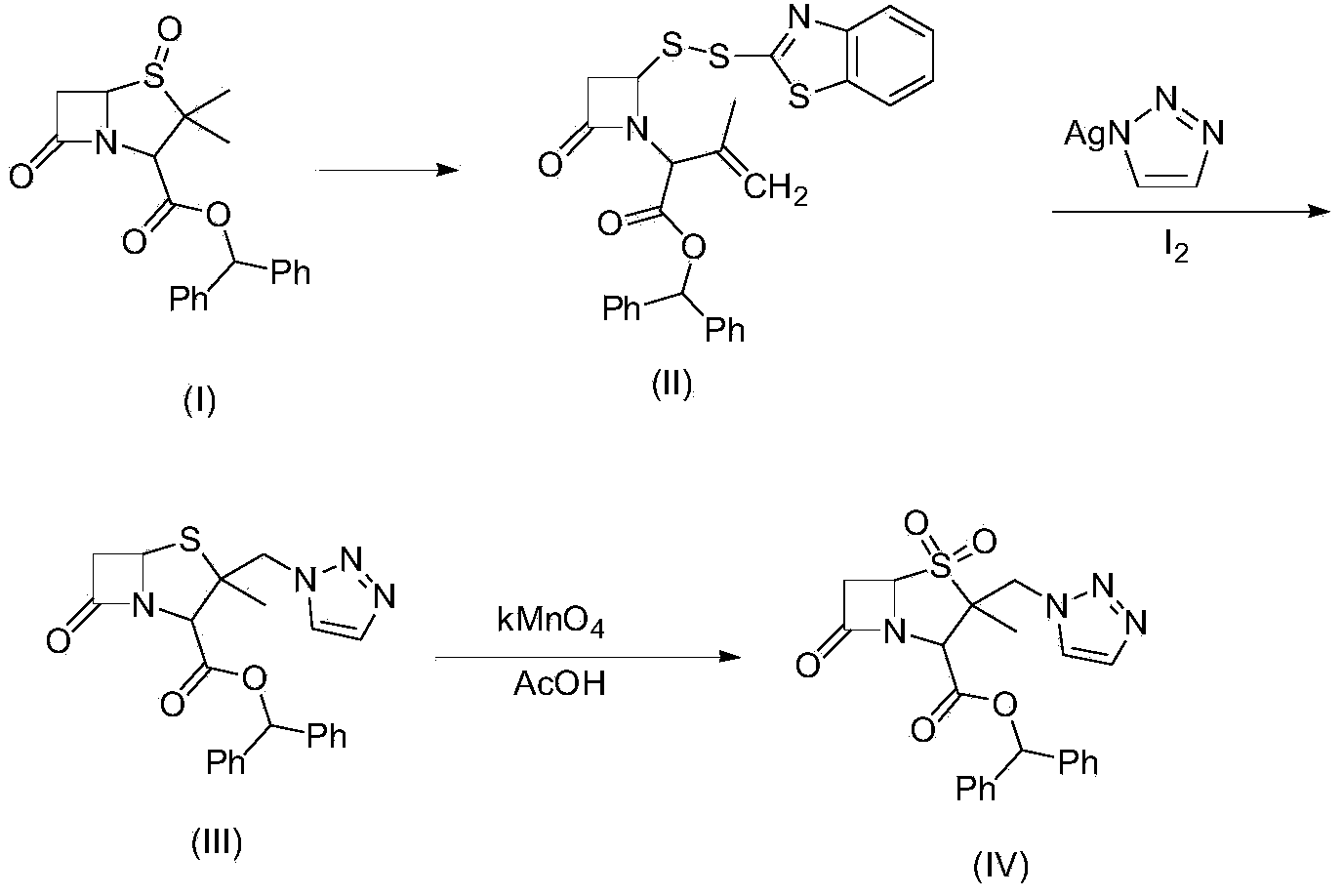
[0031] Example 1, compound (II) 3-methyl-[2-oxo-4-(2-benzothiazole dithio)-1-azetidinyl]-3-butene diphenylmethyl ester preparation:
[0032] In a 1000ml four-neck flask, put 57.5g of benzyl penicillanic acid sulfoxide, that is, compound (I), 550ml of toluene, and 26.0g of 2-mercaptobenzothiazole, raise the temperature to 60-112°C, and the reaction time is 2-3 hours , steam the azeotrope of toluene and water under normal pressure, and when the internal temperature reaches 112°C, the toluene is recovered under reduced pressure to dryness, and 75.8 g of the product is obtained, with a yield of 95%. Add 600 ml of dichloromethane and proceed to the next step of reaction.
Embodiment 2
[0033] Example 2, preparation of compound (Ⅲ) 2β-bromomethyl-2α-methyl-6,6-dihydropenicillanic acid benzhydryl ester:
[0034] Add the dichloromethane-containing compound (II) material solution of Example 1 into a 1000ml four-neck flask, then add 33.5g of copper bromide, start stirring and cool down to 5-10°C, react for 8.0h, follow by HPLC. Filter, add 2%NaHCO 3 Wash with 200ml, then wash with 200ml of water, separate the organic phase, and transfer to the next reaction.
Embodiment 3
[0035] Embodiment 3, namely the preparation of compound (Ⅳ) 2α-methyl-2β-(1,2,3,-triazol-1-yl) methyl penicillane-3α-carboxylate benzhydryl ester:
[0036] Add 50g of anion resin, 20g of 1-H-1,2,3-triazole and 60ml of ethanol to the dichloromethane phase containing compound (Ⅲ) in Example 2 in a 1000ml four-necked flask, and stir at 20-25°C 6-8.0h, HPLC tracking, after the reaction is complete, filter, add 150ML water to the anion resin and filtrate, separate layers, wash the organic phase with 100ml water, combine the organic phase to recover dichloromethane under reduced pressure, add 100ml methanol, recrystallize, filter, Dry at 40-50°C under reduced pressure to obtain 46.0 g of material, HPLC ≥ 95%, molar yield 70% (calculated based on compound Ⅱ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com