Photosensitive resin composition
A technology of photosensitive resin and composition, applied in the field of photosensitive resin composition, can solve the problem of not being able to generate alkali, etc., and achieve the effect of effective photosensitivity and excellent deep curability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
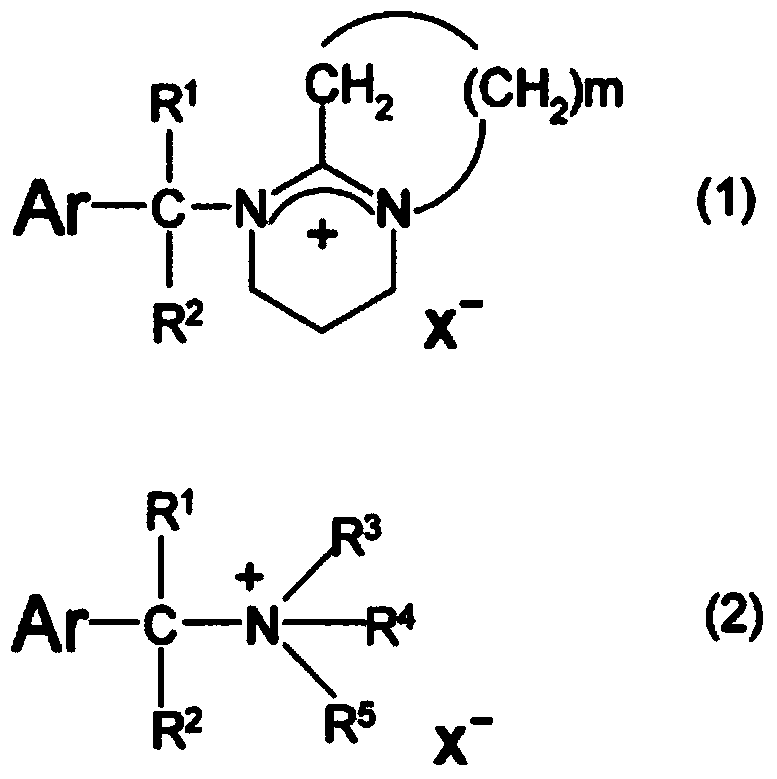
[0072] Hereinafter, the present invention will be specifically described by way of examples and production examples, but the present invention is not limited thereto. Hereinafter, parts represent parts by weight.
[0073] [Manufacture of photobase generator (A)]
manufacture example 1
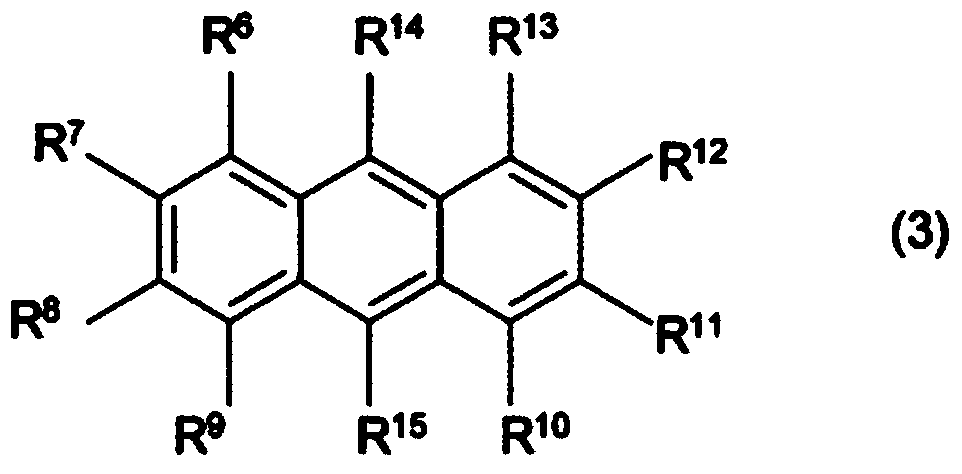
[0075] Synthesis of 8-(9-anthracenemethyl)-1,8-diazabicyclo[5.4.0]-7-undecenium tetraphenylborate (A1-1)
[0076] In a 50 ml eggplant-shaped flask, 2.0 g of 9-chloromethylanthracene (Aldrich Co.) was dissolved in chloroform, and 1.3 g of 1,8-diazabicyclo[5.4.0]-7-deca One ene (it was found to have a certain degree of heat generation after the addition), directly stirred at room temperature (about 25°C) for 1 hour to obtain a reaction solution. Into the aqueous solution consisting of 4.0 g of sodium tetraphenylborate and 40 g of water added to a 100 ml eggplant-shaped flask, the obtained reaction liquid was added drop by bit, and then stirred at room temperature (about 25 ° C) for 1 hour, Then, the aqueous layer was removed by liquid separation, and the organic layer was washed with water three times. The organic layer was concentrated by an evaporator to obtain 5.4 g of a white solid. This white solid was recrystallized with acetonitrile, and 4.7 g of photobase generators (A...
manufacture example 2
[0078] Synthesis of 1-(9-anthracenemethyl)-1-azabicyclo[2.2.2]octanium tetraphenylborate (A1-2)
[0079] Except changing "1.3 g of 1,8-diazabicyclo[5.4.0]-7-undecene" to "1.0 g of 1-azabicyclo[2.2.2]octane", by making In the same way as Example 1, 4.4g of photobase generator (A1-2) (white solid) was obtained. pass 1 The result of H-NMR analysis is {300MHz, DMSO-d6, δ (ppm): 8.9 (s, 1H), 8.7 (d, 2H), 8.2 (d, 2H), 7.7 (t, 2H), 7.6 (t , 2H), 7.3-7.1(m, 8H), 7.0-6.9(m, 8H), 6.9-6.8(m, 4H), 5.6(s, 2H), 3.6-3.4(m, 6H), 1.9(m , 1H), 1.8-1.6(m, 6H)}. This white solid was confirmed to be 1-9-anthracenemethyl-1-azabicyclo[2.2.2]octanium tetraphenylborate (A1-2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com