Method for removing NOx and SOx from fluid catalytic cracking (FCC) flue gas
A flue gas removal technology, applied in chemical instruments and methods, separation methods, air quality improvement, etc., can solve problems such as heat exchanger corrosion, blockage, serious corrosion problems, etc., to prevent the generation of escaped ammonia, Guaranteed denitrification efficiency and improved absorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
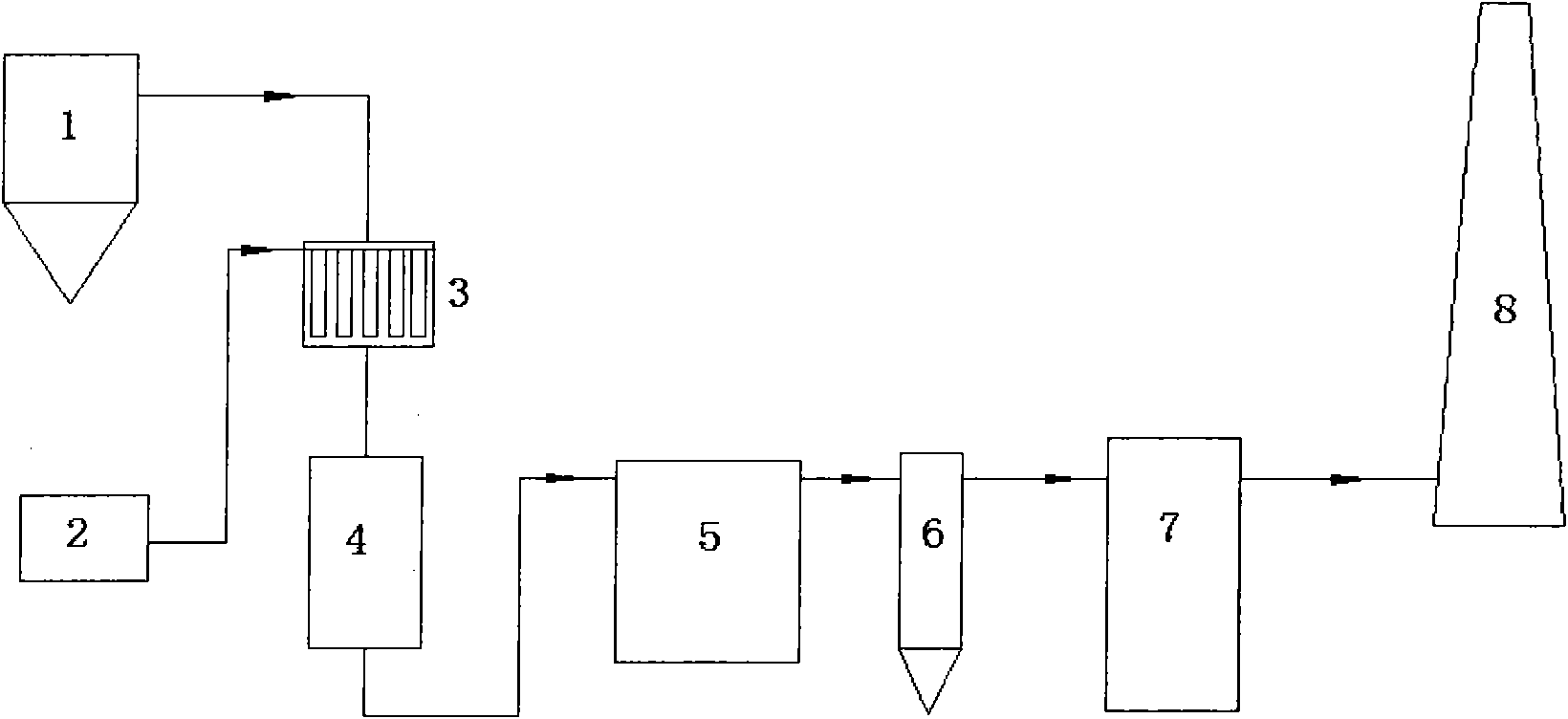
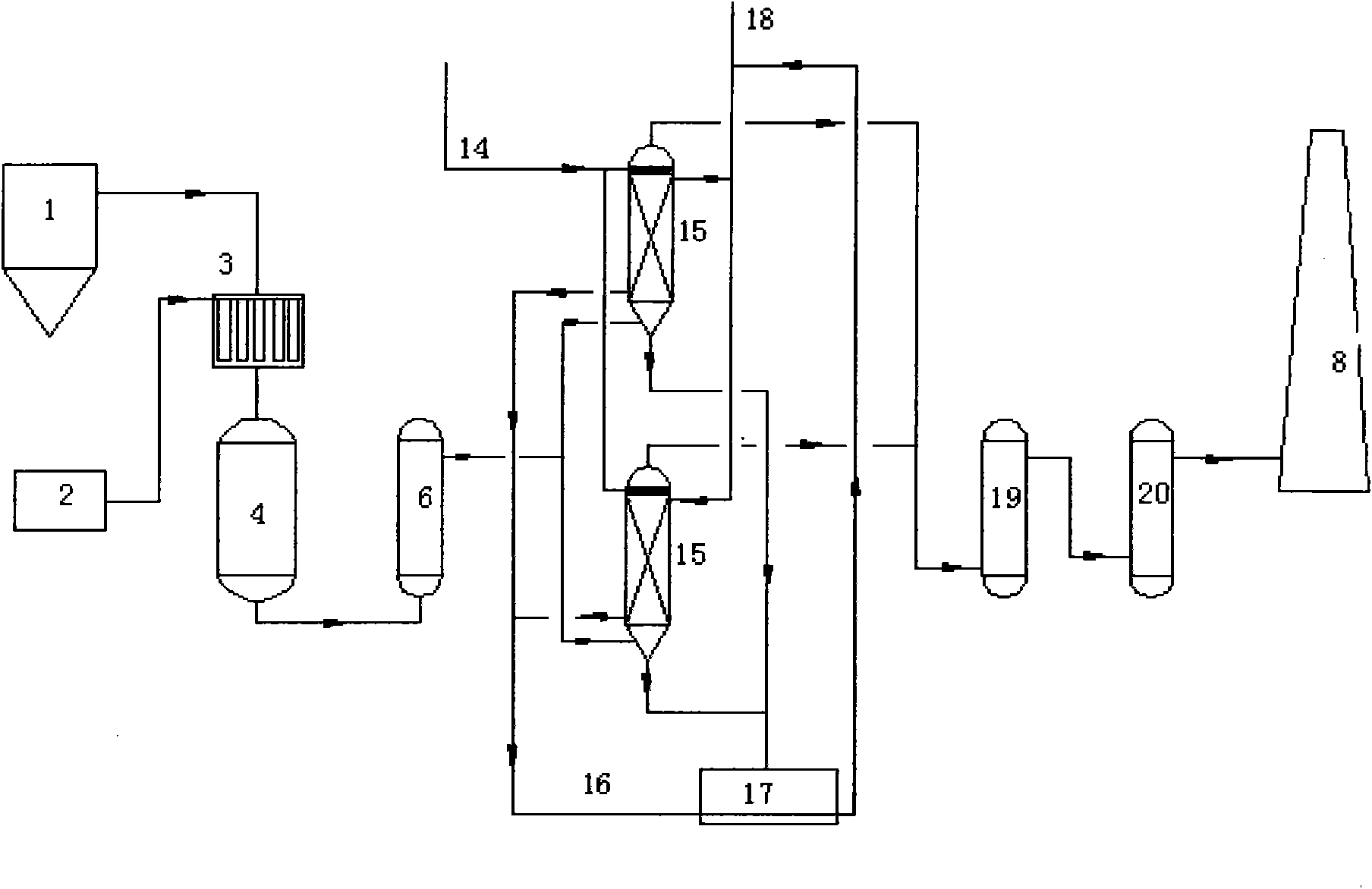
[0043] Embodiment 1 (see figure 2 )
[0044] Flue gas from FCC boiler system 1, air volume 6m 3 / h, temperature 300℃~400℃, containing 1000mg / m 3 NO x , 1000mg / m 3 SOx enters the flue gas-ammonia gas mixer 3, where the flue gas is fully and evenly mixed with the air-ammonia gas mixture from the ammonia supply unit 2, and the ammonia gas-air mixture gas volume is 0.2m 3 / h (Ammonia consumption is NO in flue gas x and SOx complete reaction required amount), after mixing with air, then enters the catalytic reduction reactor 4 downwards, the catalyst adopts the catalyst of CN200910204252.5, and this catalyst has good catalytic activity to SCR reaction.
[0045] In the catalyst bed, NO x and NH 3 N 2 and H 2 O, a soot blower is installed between the beds of the catalytic reduction reactor, and the fly ash adsorbed on the surface of the catalyst is blown back by air and then enters the dust collector to be removed. The dust collector adopts electrostatic dust removal.
[...
Embodiment 2
[0047] Embodiment 2 (see figure 2 )
[0048] Flue gas from FCC boiler system 1, air volume 30m 3 / h, temperature 300℃~400℃, containing 600mg / m 3 NO x , 2000mg / m 3 SOx enters the flue gas-ammonia gas mixer 3, where the flue gas is fully and evenly mixed with the air-ammonia gas mixture from the ammonia supply unit 2, and the ammonia-air gas mixture volume is 1m 3 / h (Ammonia consumption is NO in flue gas x and SOx complete reaction required amount), after mixing with air, then enters the catalytic reduction reactor 4 downwards, the catalyst adopts the catalyst of CN200910204252.5, and this catalyst has good catalytic activity to SCR reaction. The dust collector adopts bag dust removal, and the filter aperture is 1 to 5 mesh.
[0049] In the catalyst bed, NO x and NH 3 N 2 and H 2 O, a soot blower is installed between the beds of the catalytic reduction reactor, and the fly ash adsorbed on the surface of the catalyst is blown back by air and then enters the dust colle...
Embodiment 3
[0051] Embodiment 3 (see figure 2 )
[0052] Flue gas from FCC boiler system 1, air volume 500m 3 / h, temperature 300℃~400℃, containing 500mg / m 3 NO x , 1000mg / m 3 SOx enters the flue gas-ammonia gas mixer 3, where the flue gas is fully and evenly mixed with the air-ammonia gas mixture from the ammonia supply unit 2, and the ammonia-air gas mixture volume is 15m 3 / h (Ammonia consumption is NO in flue gas x and SOx complete reaction required amount), after mixing with air, then enters the catalytic reduction reactor 4 downwards, the catalyst adopts the catalyst of CN200910204252.5, and this catalyst has good catalytic activity to SCR reaction.
[0053] In the catalyst bed, NO x and NH 3 N 2 and H 2 O, a soot blower is installed between the beds of the catalytic reduction reactor, and the fly ash adsorbed on the surface of the catalyst is blown back by air and then enters the dust collector to be removed. The dust collector adopts bag dust removal, and the filter ape...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com