Lithium secondary battery with metal fluoride as positive electrode material
A technology for lithium secondary batteries and positive electrode materials, which is applied in secondary batteries, battery electrodes, circuits, etc., can solve the problems of low energy consumption, complicated preparation process, and difficult realization in the preparation process, and achieve simple preparation methods and high Coulombic efficiency , The effect achieved by the reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
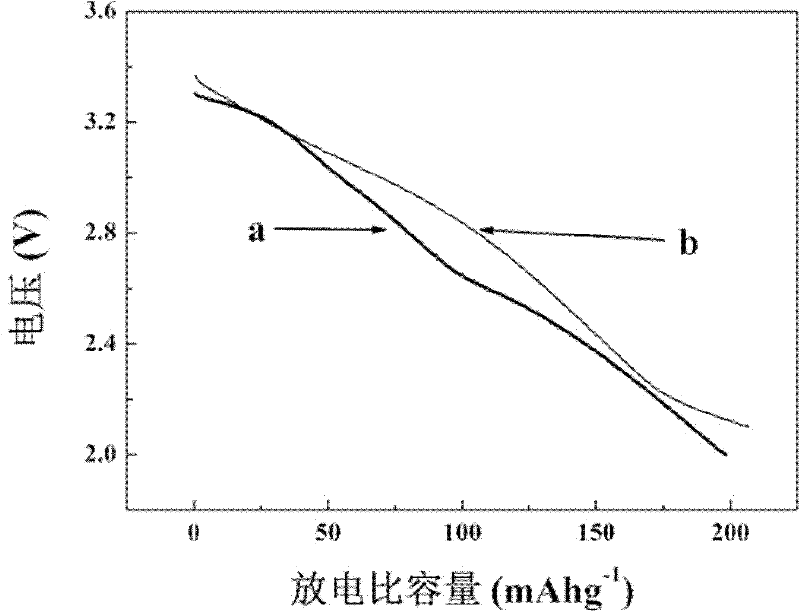
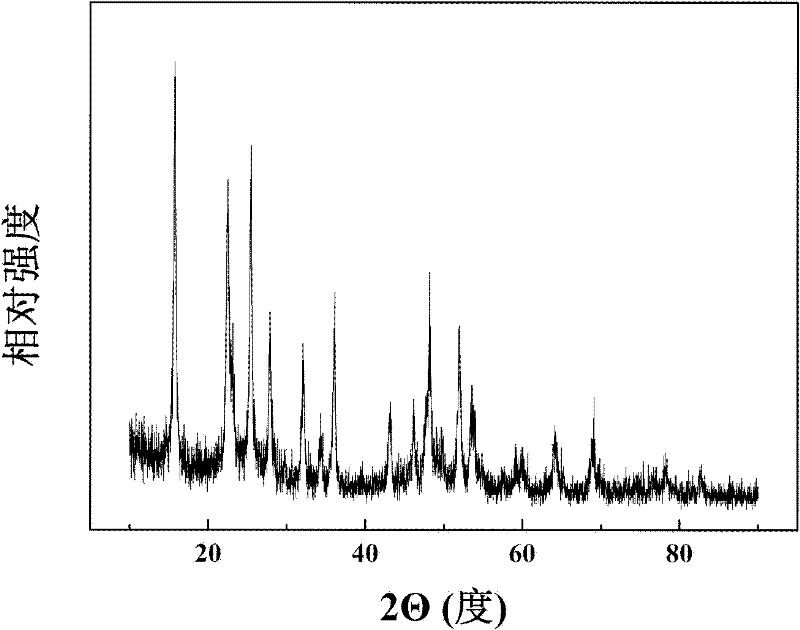
Image
Examples
Embodiment 1
[0034] Take 0.1molL -1 Fe(NO 3 ) 3 50ml of the solution was placed in a flask, and then the 60ml concentration was 0.3molL -1Aqueous ammonia was slowly added into the flask (the molar ratio of base to metal salt was 3.6:1). During this process, the solution in the flask was ultrasonically oscillated, and precipitation appeared. After the reaction was completed, the ultrasonic oscillation was stopped, and then the precipitate was obtained by suction filtration. The precipitate was first washed twice with deionized water, and then washed once with methanol. The washed precipitate was dried in vacuum at 120° C. for 1 h to obtain ferric hydroxide.
[0035] Place the ferric hydroxide in a container made of polytetrafluoroethylene, then quickly add 12ml of 40% HF in mass fraction (the molar ratio of ferric hydroxide to hydrofluoric acid is 1: 10), and seal it the container. During this process, the solution in the container was magnetically stirred and kept in a constant temper...
Embodiment 2
[0039] Take 0.1molL respectively -1 CuCl 2 Solution 20ml and 0.1molL -1 FeCl 3 Solution 80ml, placed in a flask, then 100ml concentration is 0.3molL -1 Slowly add the KOH solution in the flask (the mass ratio of alkali to metal salt is 3: 1), during this process, the solution in the flask is mechanically stirred, and precipitation occurs. After the reaction is over, stop stirring, and then suction filter to obtain the precipitate. , the precipitate was washed twice with deionized water and then once with acetone. The washed precipitate was dried in vacuum at 40° C. for 12 h to obtain a mixture of ferric hydroxide and copper hydroxide.
[0040] The ferric hydroxide and copper hydroxide mixture is placed in the container of polytetrafluoroethylene material, then the HF solution of 20ml mass fraction 40% is added wherein rapidly (the material of ferric hydroxide and copper hydroxide mixture and hydrofluoric acid The volume ratio is 1:10), and seal the container. During this ...
Embodiment 3
[0044] Take 0.1molL -1 FeCl 3 Solution 100ml, placed in a flask, then 150ml concentration is 0.3molL -1 The KOH solution was slowly added into the flask (the molar ratio of alkali to metal salt was 4.5:1), and during this process the solution in the flask was magnetically stirred, and precipitation occurred. After the reaction was over, the magnetic stirring was stopped, and then suction filtered to obtain Precipitation, the precipitate was washed once with methanol, and then washed twice with distilled water. The precipitate was dried in vacuum at 120°C for 1 h to obtain ferric hydroxide.
[0045] The ferric hydroxide was placed in a container made of polytetrafluoroethylene, and then 20ml of 40% HF solution by mass fraction was quickly added therein (the molar ratio of ferric hydroxide to hydrofluoric acid was 1:9), and Seal the container. During this process, the solution in the container was magnetically stirred and kept in a constant temperature water bath of 60°C. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com