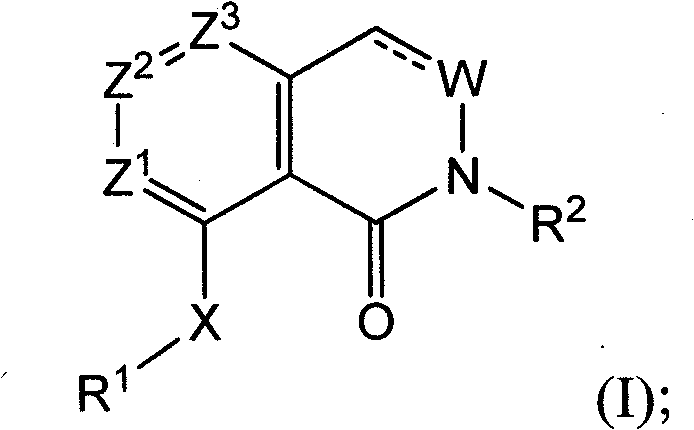
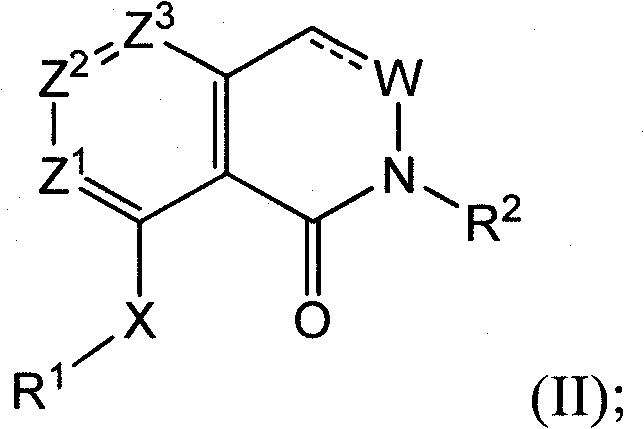
Triazolopyridines as phosphodiesterase inhibitors for treatment of dermal diseases
A compound, alkyl technology, applied in the direction of anti-inflammatory agents, drug combinations, antiviral agents, etc., can solve problems such as reducing and extending the lifespan of wild-type cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0441] Example 1 N-(1-oxo-2-(3-(trifluoromethyl)phenyl)-1,2,3,4-tetrahydroisoquinolin-8-yl)pyrazine-2-methan Preparation of Amide (Compound 101):
[0442] Step 1) Synthesis of 2-methyl-6-nitrobenzoic acid methyl ester (2):
[0443]
[0444] Dissolve 2-methyl-6-nitrobenzoic acid (1; 10 g, 55.2 mol) together with methyl iodide (17.2 mL, 276.0 mmol) and anhydrous potassium carbonate (38.1 g, 276.0 mmol) in 200 mL of methyl in ethyl ketone. The reaction mixture was stirred at reflux for 18 hours. It was then cooled to room temperature and filtered. The filtrate was diluted with EtOAc (300 mL), washed with water (2x50 mL), brine, dried (Na 2 SO 4 ), and concentrated under reduced pressure to obtain 10.80 g of 2-methyl-6-nitrobenzoic acid methyl ester 2. MS (ESI): C 9 h 9 NO 4 Calculated: 195.05; Measured 196 [M+H].
[0445] Step 2) Synthesis of 2-(bromomethyl)-6-nitrobenzoic acid methyl ester (3):
[0446]
[0447] Methyl 2-methyl-6-nitrobenzoate (2; 10.8g, 55.2mmo...
Embodiment 2
[0469] Example 2 Preparation of 8-amino-2-(3-(trifluoromethyl)phenyl)phthalazin-1(2H)-one (compound 103):
[0470] Step 1) Synthesis of 8-nitro-2-(3-(trifluoromethyl)phenyl)phthalazin-1(2H)-one (10):
[0471]
[0472] Dissolve methyl 2-formyl-6-nitrobenzoate (4; 500 mg, 2.40 mmol) in 3 mL of 1,4-bis Alkanes, 1 mL of glacial acetic acid and 3-trifluoromethylphenylhydrazine (20; 420 mg, 2.40 mmol). The reaction mixture was stirred at 200°C for 20 minutes in a microwave reactor. After cooling to room temperature, the reaction mixture was diluted with EtOAc and washed with dilute NaHCO 3 Wash with aqueous solution. The organic layer was dried (Na 2 SO 4 ), and concentrated under reduced pressure to obtain 720 mg of 8-nitro-2-(3-(trifluoromethyl)phenyl)phthalazin-1(2H)-one 10. MS(ESI)C 16 h 8 f 3 N 3 o 3 Calculated: 335.05; Measured: 336 [M+H].
[0473] Step 2) Synthesis of 8-amino-2-(3-(trifluoromethyl)phenyl)phthalazin-1(2H)-one (11):
[0474]
[0475] 8-Nitro...
Embodiment 3
[0481] Example 36-(2,3-dihydroxypropoxy)-N-(4-oxo-3-(3-(trifluoromethyl)phenyl)-3,4-dihydrophthalazine-5- base) the preparation of pyridine-2-carboxamide (compound 105):
[0482] Step 1) Synthesis of 6-((2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)pyridine-2-carboxylic acid (14):
[0483]
[0484] To a mixture of NaH (7.12 g, 178 mmol, 60% in oil) in anhydrous THF (400 mL) was added solketal (13; 23.5 g, 178 mmol) at 0°C. The mixture was stirred for 1 hour. 6-bromopyridine-2-carboxylic acid (12; 12.0 g, 59.4 mmol) was added, and stirred under reflux for 1.5 hours. Water (50 mL) was added and the pH was adjusted to 2-3. The mixture was extracted with EtOAc (4x50 mL). The combined organic layers were washed with water (3x25mL), washed with Na 2 SO 4 Drying and concentration in vacuo gave 6-((2,2-dimethyl-1,3-dioxolan-4-yl)methoxy)pyridine-2-carboxylic acid 14 as a white solid (10.0 g , 66% yield). MS(ESI)C 12 h 15 NO 5 Calculated: 253; Measured: 254 [M+H].
[0485] Ste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com