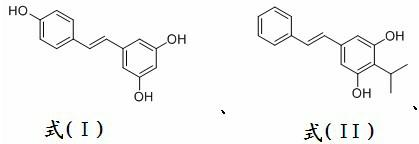
Preparation method of water-soluble toluylene compound prodrugs
A technology of stilbene and stilbene, which is applied in the field of preparation of compound prodrugs, can solve incomplete problems, achieve a wide range of applications, improve water solubility and stability, and have simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation method of PEG2000-succinyl-lysine-resveratrol prodrug
[0047] This embodiment includes the following steps:
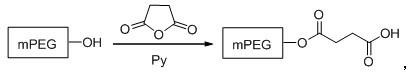
[0048] ① Preparation of PEG2000 Carboxylic Acid Derivatives
[0049] The reaction formula is as follows:
[0050]
[0051] Take 20 g (10 mmol) of dry PEG, dissolve it in 80 mL of chloroform, add 2.5 g (25 mmol) of succinic anhydride, stir well, add 2 mL of pyridine (Py), heat to reflux, react for 36 h, evaporate the solvent, The residue was washed with 60 mL saturated NaHCO 3 The solution was dissolved, extracted with ethyl acetate (20 mL×3), the aqueous phase was cooled to 0~5 ℃, adjusted to pH 2~3 with 1 mol / L hydrochloric acid, stirred for 20 min, extracted with dichloromethane (20 mL×4), combined The organic phase was neutralized by washing with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized from anhydrous ether to obtain 18.04 g of white solid PEG2000-succinic acid,...
Embodiment 2
[0066] Embodiment 2 The preparation method of PEG2000-succinyl-alanine-oxidized resveratrol prodrug
[0067] The specific operation steps are as follows:
[0068] ① The preparation methods of PEG2000 carboxylic acid derivatives and PEG2000 active esters are the same as those in Example 1;
[0069] ② Preparation of alanine-linked PEG2000 chemical modifier
[0070] Reaction equation:
[0071]
[0072] Dissolve 2 g (0.835 mmol) of PEG2000 active ester in 20 mL of DMF, then cool down to 0 to 5 °C, add alanine in NaHCO dropwise 3 solution (0.30 g alanine dissolved in 3 mL 1 mol / L NaHCO 3 solution), reacted at room temperature for 24 h, the reaction was completed, dissolved with 1 mol / L hydrochloric acid, extracted with dichloromethane (10 mL×3), combined the organic layers, washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered , concentrated, and recrystallized with anhydrous ether to obtain 1.21 g of PEG2000 chemical modifier with a yiel...
Embodiment 3
[0079] Example 3 Preparation method of PEG6000-succinyl-glycine-pterostilbene prodrug
[0080] ① Preparation of PEG6000 Carboxylic Acid Derivatives
[0081]
[0082] Dissolve 30 g (0.005 mol) of dry PEG6000 in 100 mL of chloroform, add 1.5 g (0.015 mol) of succinic anhydride, stir well, add 2 mL of pyridine (Py), heat to reflux, react for 36 h, evaporate the solvent, The residue was washed with 80 mL saturated NaHCO 3 The solution was dissolved, extracted with ethyl acetate (20 mL×3), the aqueous phase was cooled to 0~5 ℃, adjusted to pH 2~3 with 1 mol / L hydrochloric acid, stirred for 20 min, extracted with dichloromethane (20 mL×4), combined The organic phase was washed with saturated brine until neutral, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and recrystallized with anhydrous ether to obtain 26.04 g of white solid PEG6000-succinic acid with a yield of 84.0%;
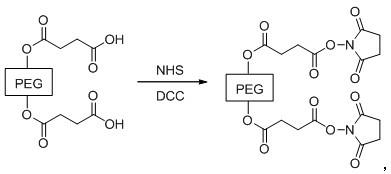
[0083] ② Synthesis of PEG6000 Active Ester
[0084]
[0085] Dissolve 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com