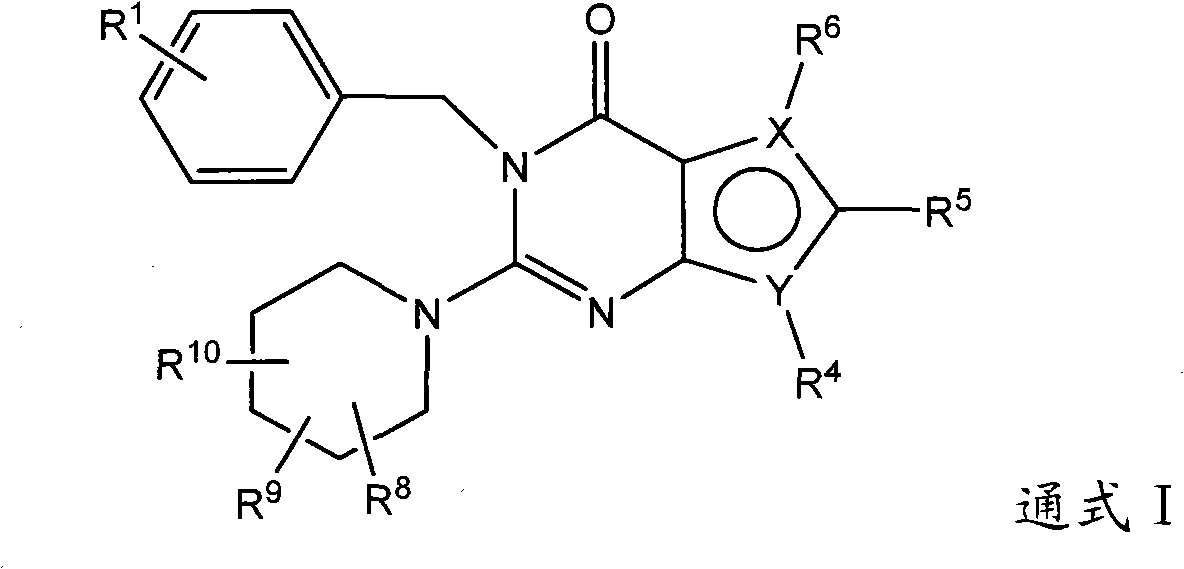
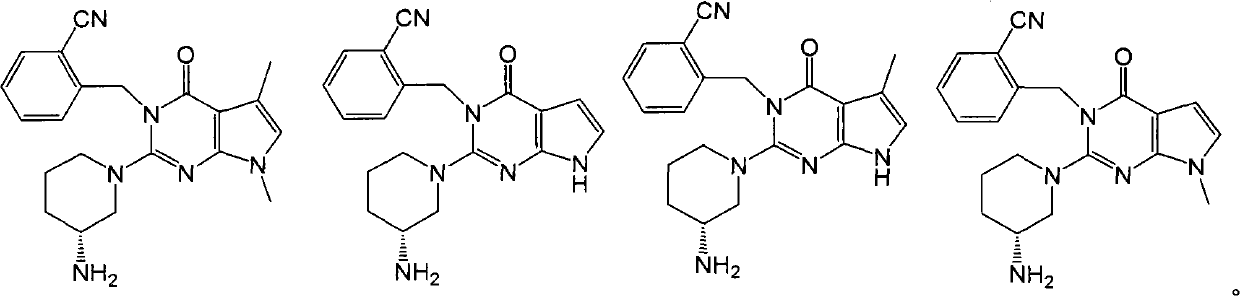
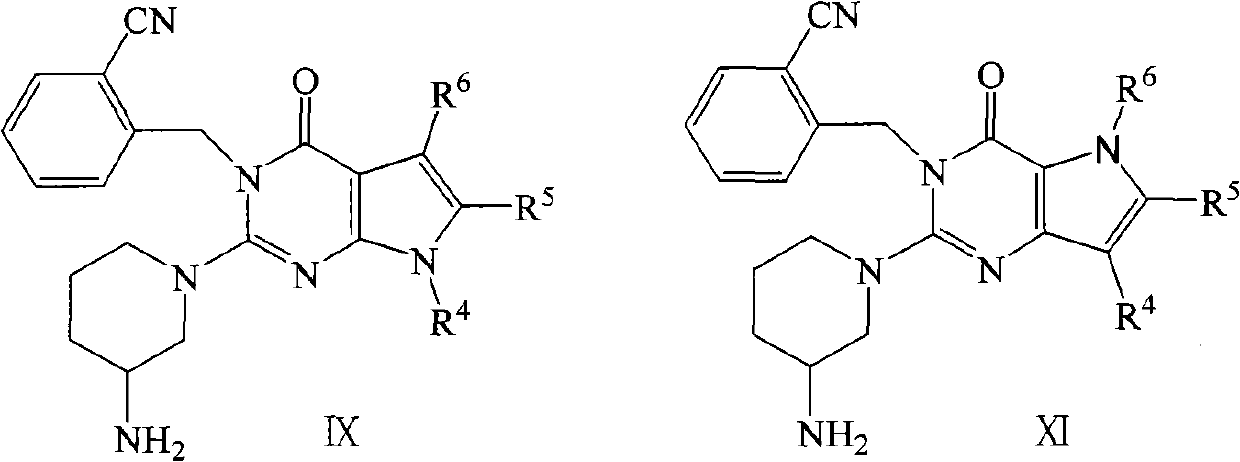
Pyrrolopyrimidone dipeptidyl peptidase-IV (DPP-IV) inhibitors
A technology of solvates and compounds, applied in the field of medicine, can solve problems such as inactivation and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1. Synthesis of compound 1
[0085]
[0086] synthetic route:
[0087]
[0088] Synthesis of Compound 1-2
[0089] Urea (1mol, 60g) was added to a 250ml dry single-necked round bottom flask, heated to 160°C in an oil bath to melt, and (0.13mol, 20g) methyl 3-aminothiophene-2-carboxylate was added, and the mixture was heated at 190- Heat and react at 200°C for 3 hours, cool, add 500ml of 10% aqueous sodium hydroxide solution, stir evenly, filter with suction, wash with 5-10% aqueous sodium hydroxide solution, and adjust the pH of the filtrate to 6.5 with 2N HCl solution in an ice bath. A white solid was precipitated, filtered with suction, washed with ice and water, and dried to obtain 12.5 g of a white solid with a yield of 59%.
[0090] 1 H-NMR (400MHz, d 6 -DMSO): δ6.9 (1H, d, J = 5.2Hz), 8.10 (1H, d, J = 5.2Hz), 11.60-11.1 (2H, br, s); MS: 169.1 [M+H + ].
[0091] Synthesis of Compounds 1-3
[0092] Mix the compound 1-2 (74.3mmol, 12.5g) obtai...
Embodiment 2
[0103] Embodiment 2. Synthesis of compound 2
[0104]
[0105] Compound 2-1 was used to replace compound 1-1 in Example 1, and the synthesis method was referred to Example 1 to prepare compound 2 as a light yellow solid with a yield of 45%.
[0106] 1 H-NMR (400MHz, CD 3 OD): δ1.25(1H, m), 1.75(1H, m), 1.77(1H, m), 1.96(1H, m), 2.03(1H, m), 2.33(3H, s), 2.71(1H , m), 2.81(1H, s), 2.89(1H, m), 3.21(1H, m), 3.42(2H, m), 5.55(2H, ABq), 7.08(1H, d, J=8Hz), 7.39(1H, t, J=7.6Hz), 7.56(1H, t, J=7.8Hz), 7.59(1H, s), 7.69(1H, d, J=7.6Hz); MS: 380.1[M+H + ], 402.1 [M+Na + ].
Embodiment 3
[0107] Embodiment 3. Synthesis of compound 3
[0108]
[0109] synthetic route
[0110]
[0111] Synthesis of compound 3-2
[0112] Add compound 3-1 (77.5g, 0.5mol), methyl cyanoacetate (99.1g, 1mol) and 50ml of methanol into a 250ml round bottom flask, add 1ml of DMF and 5ml of triethylamine dropwise under ice-cooling, and heat to 70°C After reacting for 3 hours, the solvent was evaporated under reduced pressure, and the residue was treated with 1 L of cold water and stirred to obtain a beige precipitate, which was filtered with suction, washed with cold water, and dried to obtain 113 g of gray solid compound 3-2 with a yield of 79.6%.
[0113] 1 H-NMR (400MHz, CDCl 3 ): δ6.98 (1H, d, J = 5.2Hz), 6.30 (1H, d, J = 5.2Hz), 4.0 (2H, s), 3.80 (3H, s); MS: 158.0 [M+H + ].
[0114] Synthesis of Compound 3-3
[0115] Dissolve the compound 3-2 (9.5g, 6mmol) obtained in the above step in 300ml of dry dichloromethane, cool to -60°C, add 9g of chlorosulfonyl isocyanate dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com