Reductively degradable mercaptopurine nanometer micellar prodrug with controllable drug release and application thereof
A technology of nano-micelle and mercaptopurine, which is applied in the field of nano-medicine and new materials, and biomedical technology, can solve the problems of unfavorable long-term circulation, lack of biocompatibility, and inability to achieve stable release, and achieve the goal of reducing drug toxicity and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
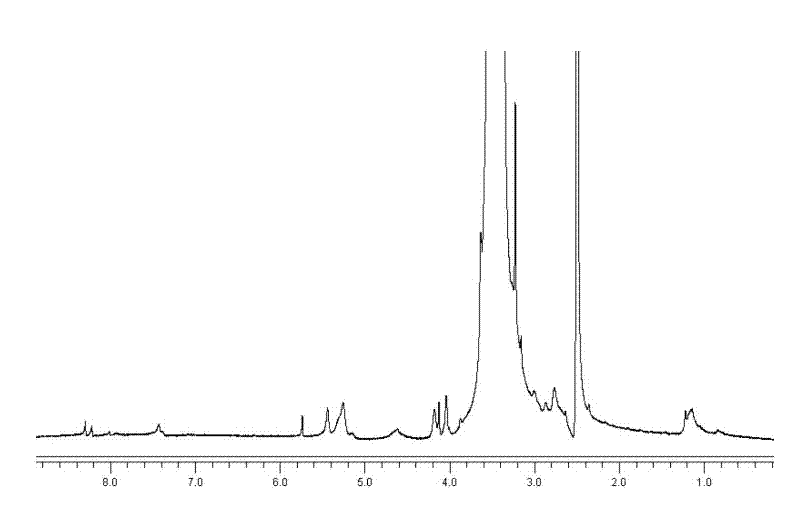
[0043] Preparation of mercaptopurine nanomicelle prodrug of the present invention
[0044] Step 1: Synthesis of mPEG-NPC
[0045] 1.1) Take mPEG (Mw= 1900, 7.6 g, 4 mmol) and p-nitrophenyl chloroformate (NPC, 3.21 g, 16 mmol) and dissolve them in 30 mL of dichloromethane (DCM);
[0046] 1.2) Add 0.81 mL (10 mmol) of pyridine to the mPEG solution in DCM, drop NPC into the mPEG solution drop by drop under the protection of nitrogen at room temperature, and stir for 24 h;
[0047] 1.3) Concentrate the reaction solution and re-precipitate in ether 3 times, and then vacuum dry to obtain mPEG-NPC.
[0048] The second step, mPEG-SS-NH 2 Synthesis
[0049] 2.1) Dissolve cystamine dihydrochloride (2.89 g, 12.88 mmol) and triethylamine (4.21 mL, 30 mmol) in dimethyl sulfoxide (DMSO), and gradually drip into the first product mPEG-NPC (3.8 g, 1.84 mmol) in DMSO solution, keep stirring for 24 hours;
[0050] 2.2) Dialysis the mixed solution with a dialysis bag with a molecular weight cut-off of 1000...
Embodiment 2
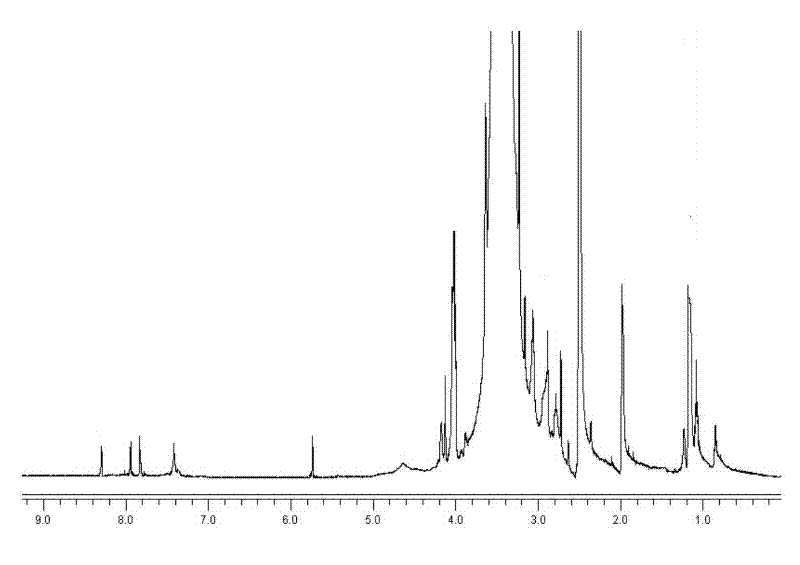
[0068] Assembly of targeted mercaptopurine nanomicelle prodrugs
[0069] Weigh 20 mg of lyophilized mPEG-SS-NH-g-PAsp-MP and dissolve it in 10 mL of deionized water. After ultrasonic vibration, amphiphilic nanomicelles are formed. The particle size measured by dynamic light scattering is 160 nm. ( Image 6 ), belongs to the nanometer level.
Embodiment 3
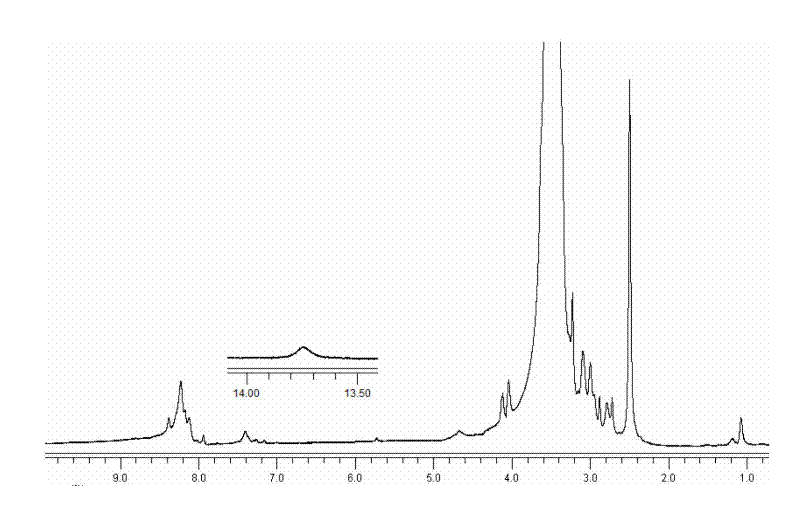
[0071] Degradation of targeted mercaptopurine nanomicelle prodrugs
[0072] Sufficient dithiothreitol (DTT) was added to the aforementioned micelles to reduce the degradation of the micelles, and 1 mL was sampled at regular intervals, and the change in particle size was measured by DLS. Such as Figure 7 It is shown that with the increase of time, the particle size of the micelles gradually increases, indicating that under the reducing action of DTT, the disulfide bonds are broken, and the micelles are degraded and agglomerated to increase the particle size.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com