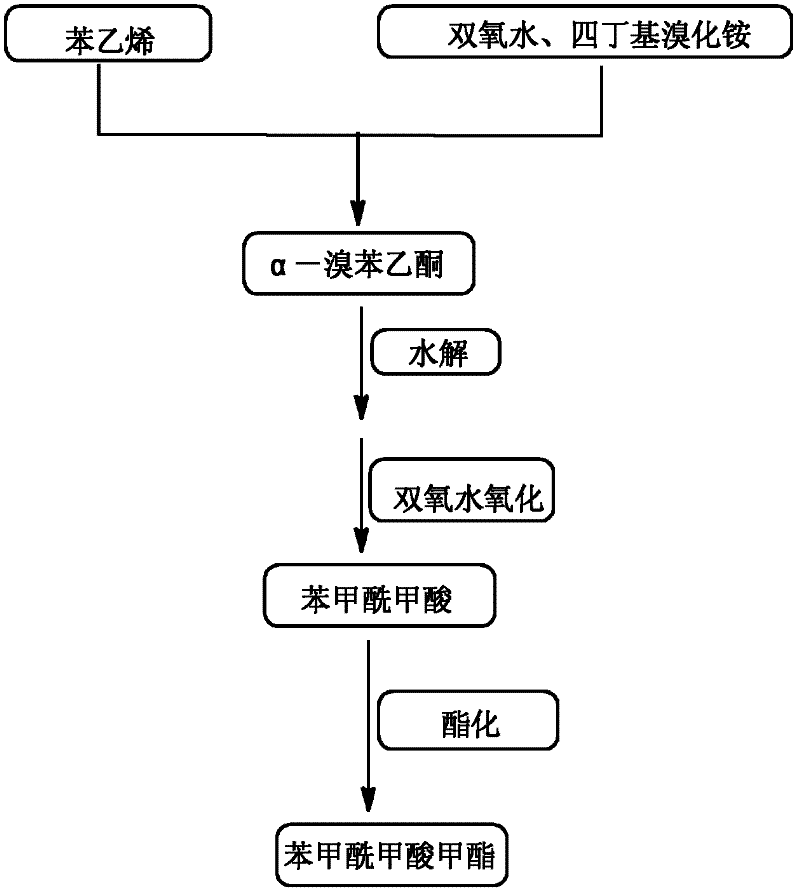
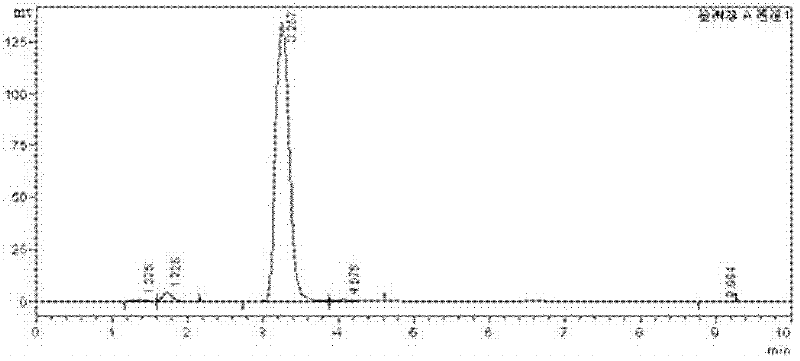
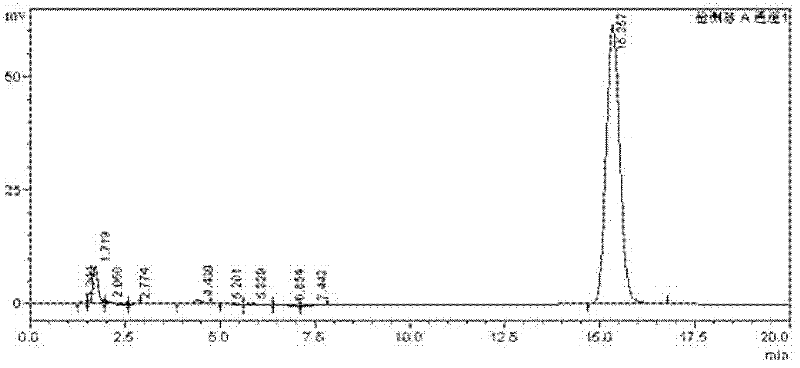
High-selectivity synthesis method of benzoyl formic acid
A technology of benzoylformic acid and a synthesis method, which is applied in the field of highly selective synthesis of benzoylformic acid, can solve problems such as unfavorable large-scale industrial production sustainable development, backward production method of benzoylformic acid, serious environmental pollution, and the like, Achieve the effect of improving product yield and purity, reducing production costs and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 150.00g of water, 10.40g of styrene and 35.46g of tetrabutylammonium bromide into a 500mL three-necked flask, add 22.67g of hydrogen peroxide solution with a mass fraction of 30% dropwise at 10-25°C under stirring, and control the dropping time to 1 -1.5h, after the dripping, raise the temperature to 80-100°C, continue the reaction for 10-13 hours, after the end of the reaction, cool down to room temperature, extract the reaction solution with ethyl acetate for 2-3 times, combine the extracts for 0.3-0.5 MPa, 40-45 ℃ decompression distillation to remove the solvent, and finally recrystallized from dichloromethane to obtain α-bromophenylethanol;
[0044] Slowly add 22.67g of 30% hydrogen peroxide solution dropwise into α-bromophenylethanol. After the dropwise addition, react at room temperature for 18-23h. After the reaction, separate the organic phase and the aqueous phase by standing still. Extract the organic phase with ethyl acetate for 2-3 times, combine the ext...
Embodiment 2
[0050] Add 150.00g of water, 10.40g of styrene and 35.46g of tetrabutylammonium bromide into a 500mL three-necked flask, add 21.53g of hydrogen peroxide solution with a mass fraction of 30% dropwise at 10-25°C under stirring, and control the dropping time to 1 -1.5h, after the dripping, raise the temperature to 80-100°C, continue the reaction for 10-13 hours, after the end of the reaction, cool down to room temperature, extract the reaction solution with ethyl acetate for 2-3 times, combine the extracts for 0.3-0.5 MPa, 40-45 ℃ decompression distillation to remove the solvent, and finally recrystallized from dichloromethane to obtain α-bromophenylethanol;
[0051] Slowly add 22.67g of 30% hydrogen peroxide solution dropwise into α-bromophenylethanol. After the dropwise addition, react at room temperature for 18-23h. After the reaction, separate the organic phase and the aqueous phase by standing still. Extract the organic phase with ethyl acetate for 2-3 times, combine the ext...
Embodiment 3
[0056] Add 150.00g of water, 10.40g of styrene and 45.08g of tetrabutylammonium bromide into a 500mL three-necked flask, add 22.67g of hydrogen peroxide solution with a mass fraction of 30% dropwise at 10-25°C under stirring, and control the dropping time to 1 -1.5h, after the dripping, raise the temperature to 80-100°C, continue the reaction for 10-13 hours, after the end of the reaction, cool down to room temperature, extract the reaction solution with ethyl acetate for 2-3 times, combine the extracts for 0.3-0.5 MPa, 40-45 ℃ decompression distillation to remove the solvent, and finally recrystallized from dichloromethane to obtain α-bromophenylethanol;
[0057] Slowly add 22.67g of 30% hydrogen peroxide solution dropwise into α-bromophenylethanol. After the dropwise addition, react at room temperature for 18-23h. After the reaction, separate the organic phase and the aqueous phase by standing still. Extract the organic phase with ethyl acetate for 2-3 times, combine the ext...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com