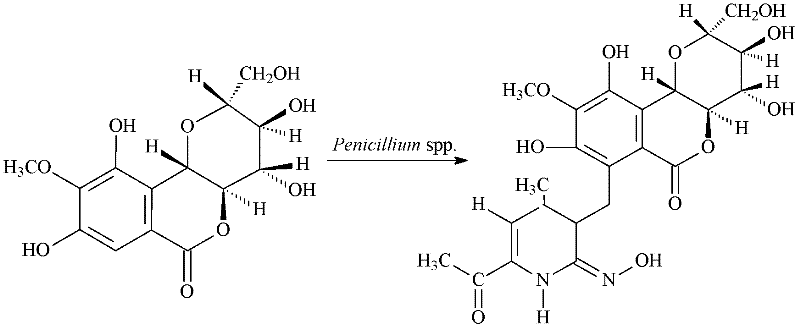
Method for converting bergenin into special nitrogenous derivative by using penicillium
A technology of petrolatum and Penicillium, which is applied in the field of using Penicillium to transform petrakinin into special nitrogen-containing derivatives, and can solve the problems of poor water solubility, insignificant activity, poor oral absorption and the like of petrolatum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
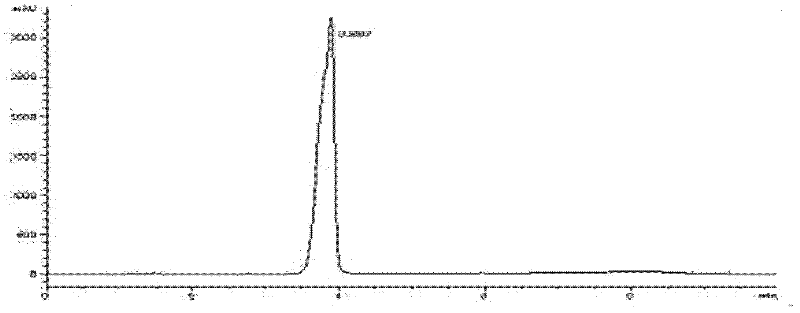
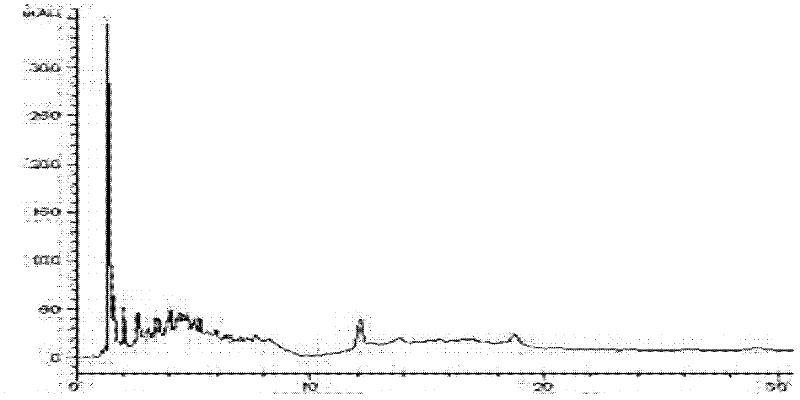
Image
Examples
Embodiment 1
[0054] Fungal fermentation medium: glucose 20 g, yeast extract 5 g, peptone 5 g, NaCl 5 g, K 2 HPO 4 5 g, distilled water 1000 mL, pH 6.5.
[0055] Pour 50mL of the fungal fermentation medium into a 250mL Erlenmeyer flask, and after autoclaving at 121°C, insert Penicillium clatices ( Penicillium crustosum , CGMCC No. 3.7142) strain, 28°C, 180r / min shake flask fermentation and culture for 48 hours, stop the fermentation, add 2% petracenin DMSO solution to the medium, 28°C, after 96 hours, stop the transformation reaction, filter to remove the mycelia, and the supernatant contains the transformation product of petogenin ( E )-7-((6-acetyl-2-(oximino)-4-methyl-1, 2-dihydropyridine-3-hydrocarbyl)methyl)-3, 4, 8, 10-tetrahydroxy- 2-(Hydroxymethyl)-9-methoxy-2, 3, 4, 4a-tetrahydro[3, 2- c ] Isochromen-6(10b H)-one. The supernatant was extracted three times with 50 mL of ethyl acetate, and then purified by silica gel column chromatography (chloroform:methanol=10:1) and Sephade...
Embodiment 2
[0057] Fungal fermentation medium: glucose 20 g, yeast extract 5 g, peptone 5 g, NaCl 5 g, K 2 HPO 4 5 g, distilled water 1000 mL, pH 6.5.
[0058] Pour 50mL of the fungal fermentation medium into a 250mL Erlenmeyer flask, and after autoclaving at 121°C, insert Penicillium clatices ( Penicillium crustosum , CGMCC No. 3.7142) strain, 28°C, 180r / min shake flask fermentation culture for 48 hours, stop the fermentation, add DMSO solution with a mass fraction of 2% petgenin in the medium, 28°C, after 120 hours, stop the transformation reaction, filter to remove the mycelia, and the supernatant contains the transformation product of petogenin ( E )-7-((6-acetyl-2-(oximino)-4-methyl-1, 2-dihydropyridine-3-hydrocarbyl)methyl)-3, 4, 8, 10-tetrahydroxy- 2-(Hydroxymethyl)-9-methoxy-2, 3, 4, 4a-tetrahydro[3, 2- c ] Isochromen-6(10b H)-one. The supernatant was extracted three times with 50 mL of ethyl acetate, and then purified by silica gel column chromatography (chloroform:methano...
Embodiment 3
[0060] Fungal fermentation medium: glucose 20 g, yeast extract 5 g, peptone 5 g, NaCl 5 g, K 2 HPO 4 5 g, distilled water 1000 mL, pH 6.5.
[0061] Pour 50mL of the fungal fermentation medium into a 250mL Erlenmeyer flask, and after autoclaving at 121°C, insert Penicillium clatices ( Penicillium crustosum , CGMCC No. 3.7142) strain, 28°C, 180r / min shake flask fermentation culture for 48 hours, stop the fermentation, add daidzein DMSO solution with a mass fraction of 2% to the medium, 28°C, after 96 hours, stop the transformation reaction, filter to remove the mycelia, and the supernatant contains the transformation product of petogenin ( E )-7-((6-acetyl-2-(oximino)-4-methyl-1, 2-dihydropyridine-3-hydrocarbyl)methyl)-3, 4, 8, 10-tetrahydroxy- 2-(Hydroxymethyl)-9-methoxy-2, 3, 4, 4a-tetrahydro[3, 2- c ] Isochromen-6(10b H)-one. After supernatant was extracted three times with 50mL ethyl acetate, after HPLC preparative column was prepared again, the petracenin transformat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com