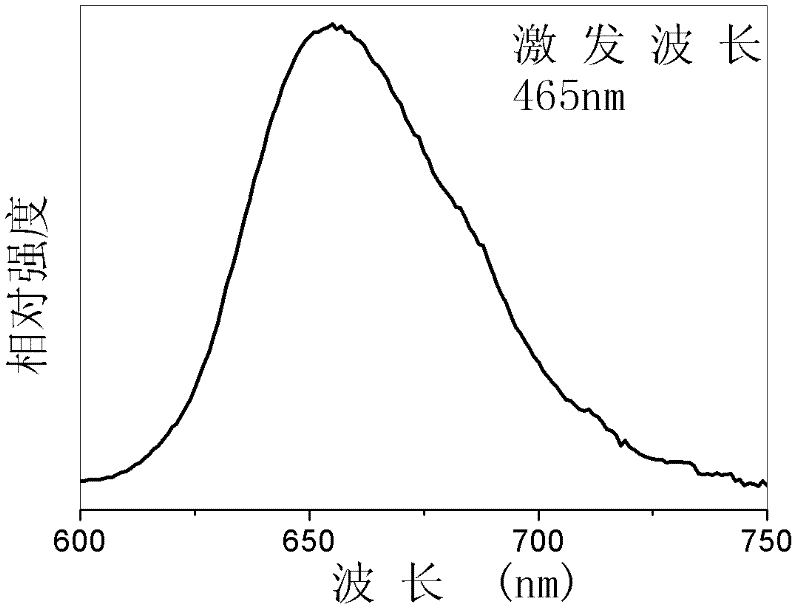
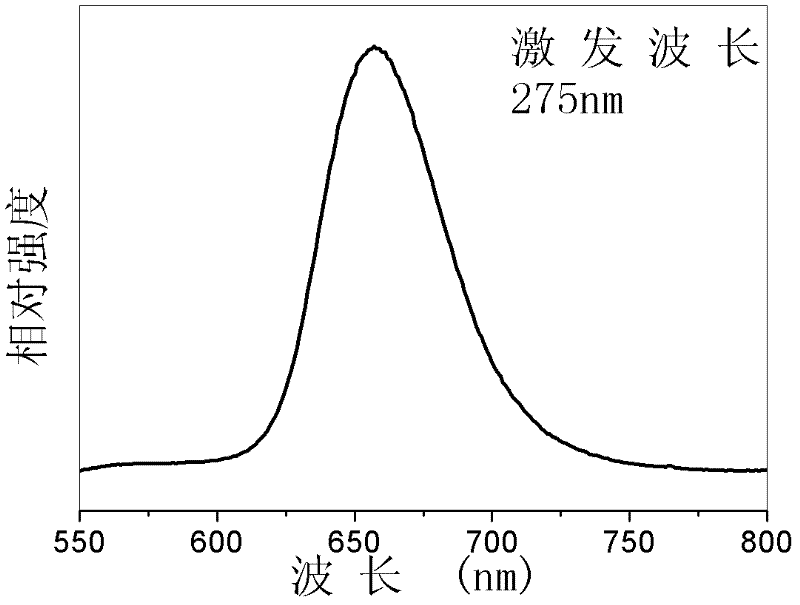
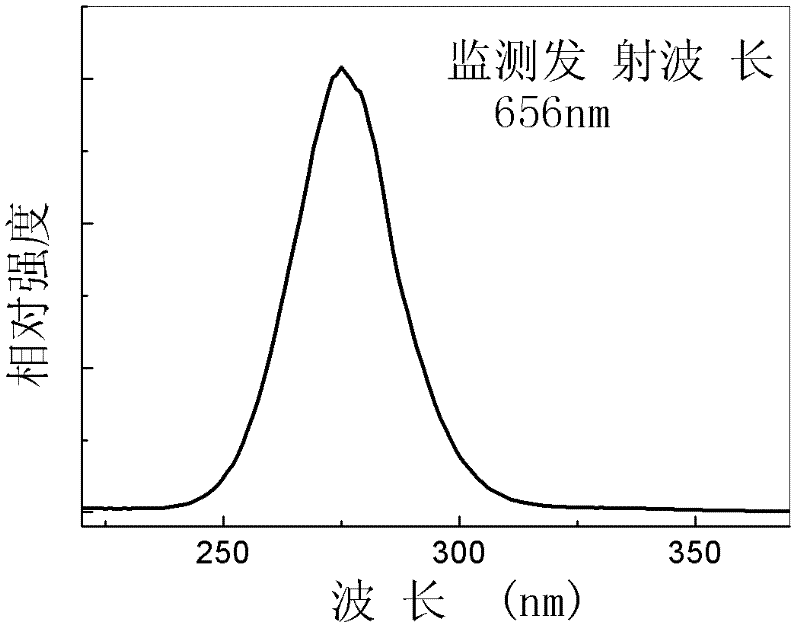
Bivalent bismuth ion-doped chloro-barium pentaborate red fluorescent material and preparation method thereof
A kind of barium chloropentaborate, red fluorescence technology, applied in the direction of luminescent materials, chemical instruments and methods, etc., to achieve the effect of improving the color rendering index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Choose barium carbonate, boric acid, barium chloride and bismuth trioxide as starting materials, press Ba 2(1-x) B 5 o 9 Cl: 2xBi (x=0.05) shows the molar ratio, namely Ba: B: Cl: Bi = 1.90: 5: 1: 0.10, four kinds of raw materials are weighed respectively, and the total weight of the control mixture is 50 grams. After 50 grams of the mixture were mixed by ball milling, they were put into a corundum crucible, and then the crucible was put into a high-temperature electric furnace. Control the heating rate, control the decomposition rate of boron compounds, and prevent the mixture from overflowing from the crucible. The sample is at 500 o C pre-fired for 10 hours. Take out the pre-fired sample, grind and mix it again, put it into a crucible, and put it into a crucible at 1000 o C, fired twice for 10 hours, and ground again in the middle. The fired samples were placed in 1000 o CH 2 The bivalent bismuth-doped red fluorescent material was prepared after being treated ...
Embodiment 2
[0022] Choose barium hydroxide, diboron trioxide, ammonium chloride and bismuth trioxide as starting raw materials, press Ba 2(1-x) B 5 o 9 The molar ratio shown by Cl:2xBi (x = 0.0001), namely Ba: B: Cl: Bi = 1.9998: 5: 1: 0.0002, respectively weighed the four raw materials, and controlled the total weight of the mixture to be 50 grams. After 50 grams of the mixture were mixed by ball milling, they were put into a corundum crucible, and then the crucible was put into a high-temperature electric furnace. Control the heating rate, control the decomposition rate of boron compounds, and prevent the mixture from overflowing from the crucible. The sample is at 400 o C pre-fired for 10 hours. The pre-fired sample is taken out, ground and mixed again, put into a crucible, and heated at 800 o C, fired twice for 10 hours, and ground again in the middle. The fired samples were placed at 800 o C N 2 +H 2 The bivalent bismuth-doped red fluorescent material was prepared after being...
Embodiment 3
[0024] Choose barium oxide, metaboric acid, barium chloride containing crystal water and bismuth powder as starting materials, press Ba 2(1-x) B 5 o 9 Cl:2xBi (x = 0.10) shows the molar ratio, namely Ba: B: Cl: Bi = 1.80: 5: 1: 0.20, respectively weigh four kinds of raw materials, and control the total weight of the mixture to be 50 grams. After 50 grams of the mixture were mixed by ball milling, they were put into a corundum crucible, and then the crucible was put into a high-temperature electric furnace. Control the heating rate, control the decomposition rate of boron compounds, and prevent the mixture from overflowing from the crucible. The sample is at 750 o C pre-fired for 5 hours. The pre-fired sample is taken out, ground and mixed again, put into a crucible, and heated at 900 o C, fired twice for 5 hours, and ground again in the middle. The fired samples were placed at 900 o C N 2 +H 2 The bivalent bismuth-doped red fluorescent material was prepared after being...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com