Primer sequences and quantitative determination kit used for simultaneously detecting human CMV and BK virus DNA
A quantitative detection and virus technology, applied in the direction of recombinant DNA technology, DNA / RNA fragments, microbial measurement / inspection, etc., to achieve the effect of simple composition, multiple experimental information, and reduction of manpower and material resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A set of primers and probes for the quantitative detection of CMV and BKV DNA. Including a pair of primers against CMV comprising upstream primers SEQ ID NO.1 and downstream primers SEQ ID NO.2 and a probe SEQ ID NO.3; and a pair of primers against BKV comprising upstream primers SEQ ID NO.4 and downstream Primer SEQ ID NO.5, and a probe SEQ ID NO.6. SEQ ID NO.3 and SEQ ID NO.6 can be labeled with different fluorescent dyes for quantitative detection.
Embodiment 2
[0032] A standard product matching the method for detecting CMV and BKV DNA, simultaneously containing the purified plasmid of CMV and BKV two kinds of detection target DNA fragments SEQ ID NO.9. The preparation process obtains the target fragment after amplifying the CMV and BKV DNA and connects it to the T vector. Use the primers of SEQ ID NO.10 and SEQ ID NO.11 to amplify the CMV DNA. The program is 95°C for 2min; 94°C for 20sec, 60°C for 30sec, and 72°C for 30sec for a total of 35 cycles. The conditions are 200nM primers and Taq enzyme 50U / mL, 100μM dNTPs, 20mM Tris-HCl, 20mM KCl, 10mM (NH 4 ) 2 SO 4 , 2mM MgSO 4 . The CMV target DNA fragment was obtained by the above method. PCR amplification of BKV DNA was performed using primers of SEQ ID NO.12 and SEQ ID NO.13. The program was 95°C for 2min; 94°C for 20sec, 60°C for 30sec, and 72°C for 30sec for a total of 35 cycles, and the condition was 200nM Primers, Taq enzyme 50U / mL, 100μM dNTPs, 20mM Tris-HCl, 20mM KCl, 10m...
Embodiment 3
[0034] A kit for quantitatively detecting the DNA of CMV and BKV, consisting of the following reagents:
[0035] Reagent A: A 250 μL solution is composed of a mixture of primers and probes for CMV and BKV. The solution contains 5 μM SEQ ID NO.1, 5 μM SEQ ID NO.2, 5 μM SEQ ID NO.4, 5 μM SEQ ID NO.5 and 2 μM probe SEQ ID NO.3, 2 μM probe SEQ ID NO.6;
[0036] Reagent B: Consists of 250 μL buffer containing 1 mM dNTPs (including dATP, dCTP, dGTP, dTTP), 200 mM Tris-HCl, 200 mM KCl, 100 mM (NH 4 ) 2 SO 4 , 45 mM MgSO 4 ;
[0037] Reagent C: 5U / μL Taq DNA polymerase;
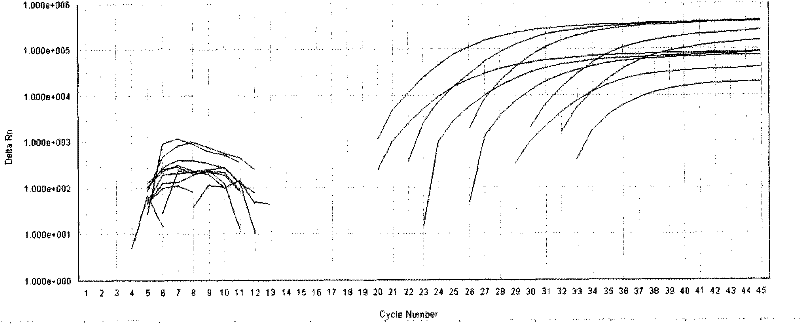
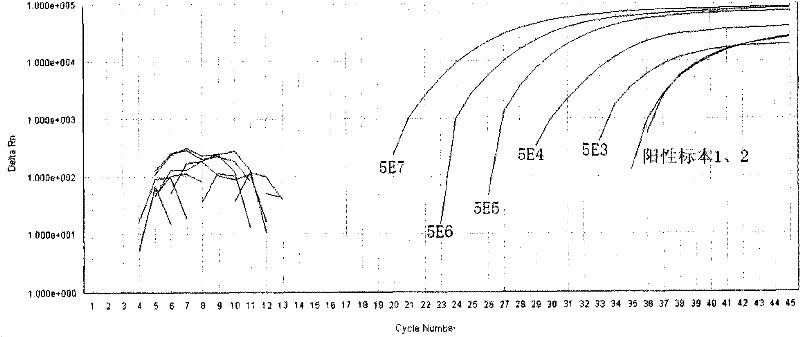
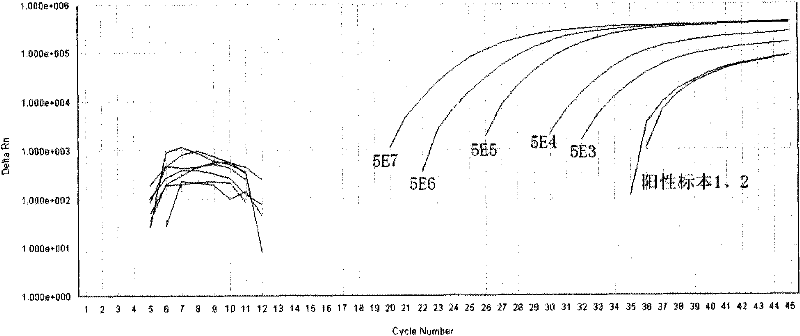
[0038] Reagent D: The prepared standard was serially diluted to reach 5E7 (5×10 7 copy / mL), 5E6, 5E5, 5E4, 5E3, used for standard curve formulation;
[0039] Reagent E: negative control (normal human DNA without CMV and BKV infection);
[0040] Reagent G: Positive control (using 5E4 standard).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com