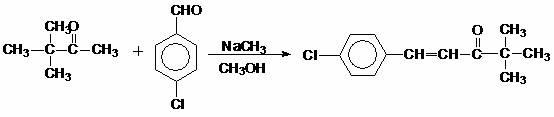Method for preparing 4,4-dimethyl-1-(4-chlorphenyl)-3-pentanone
A dimethyl and chlorophenyl technology, applied in the field of organic compound synthesis, can solve the problems of poor product quality, high equipment requirements, low synthesis yield, etc., and achieves the advantages of reducing unit consumption, reducing equipment requirements and improving reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Add 100g (0.71mol) of p-chlorobenzaldehyde, 76g (0.76mol) of 3,3-dimethyl-2-butanone, 200g of methanol, and 46g (0.86mol) of solid sodium methoxide into a 500ml reaction bottle, and heat up to reflux. The reflux temperature is 67-70°C, and it is kept at reflux for 6 hours. After the reflux is over, the recovery of methanol is started. When the recovery reaches 170g, the recovery of methanol is stopped. The product is cooled to 10-20°C, filtered, and dried at 60°C for 5 hours to obtain light Yellow crystalline solid 148g, content: 97.2% (HPLC), yield 95.1%. Recover 0.32% methanol water (mass content, the same below).
Embodiment 2
[0013] Add 100g (0.71mol) of p-chlorobenzaldehyde, 80g (0.8mol) of 3,3-dimethyl-2-butanone, 200g of methanol, and 50g (0.93mol) of solid sodium methoxide into a 500ml reaction bottle, and heat up to reflux. The reflux temperature is 67-70°C, and it is kept at reflux for 6 hours. After the reflux is over, the recovery of methanol is started. When the recovery reaches 170g, the recovery of methanol is stopped. The product is cooled to 10-20°C, filtered, and dried at 60°C for 5 hours to obtain light Yellow crystalline solid 151g, content: 96.8% (HPLC), yield 97.1%. Recover 0.25% methanol water.
Embodiment 3
[0015] Add 100g (0.71mol) of p-chlorobenzaldehyde and 83.6g (0.84mol) of 3,3-dimethyl-2-butanone into a 500ml reaction bottle, recover 200g of methanol and 54g (1mol) of solid sodium methoxide, and heat up to reflux , the reflux temperature is 67-70°C, and it is kept under reflux for 6 hours. After the reflux is over, the recovery of methanol is started. When the recovery reaches 170g, the recovery of methanol is stopped, the product is cooled to 10-20°C, filtered, and dried at 60°C for 5h to obtain 149 grams of light yellow crystalline solid, content: 96.4% (HPLC), yield 95.8%. Recover methanol water 0.33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com