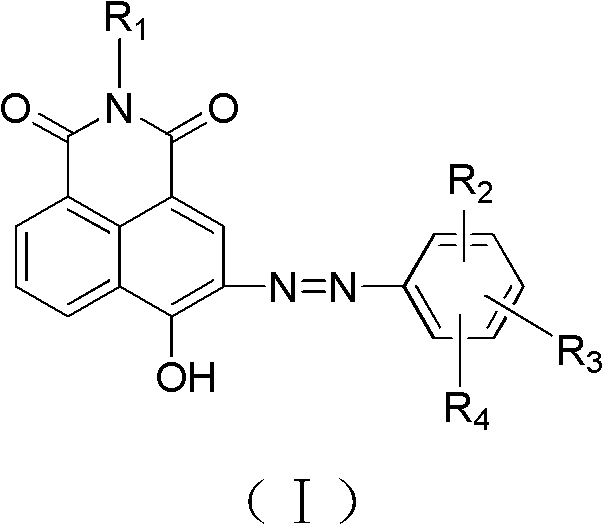
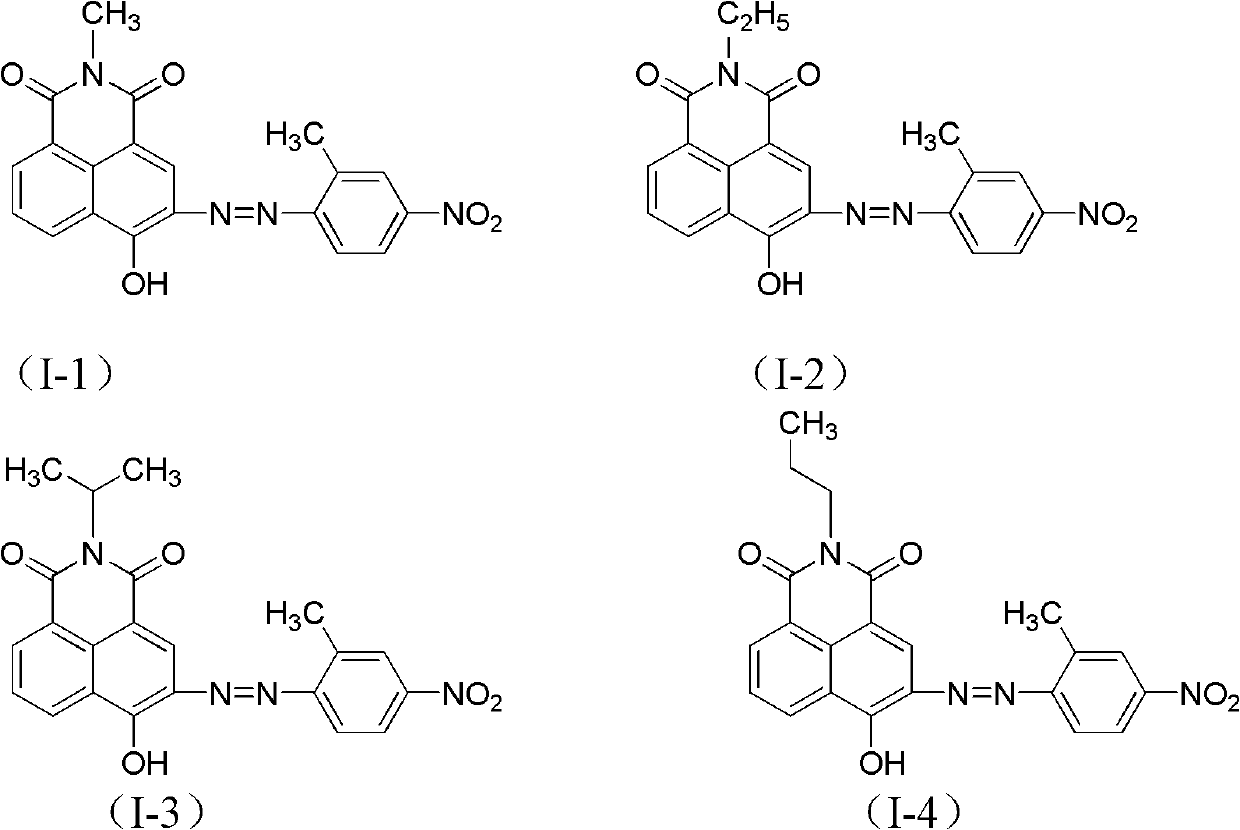
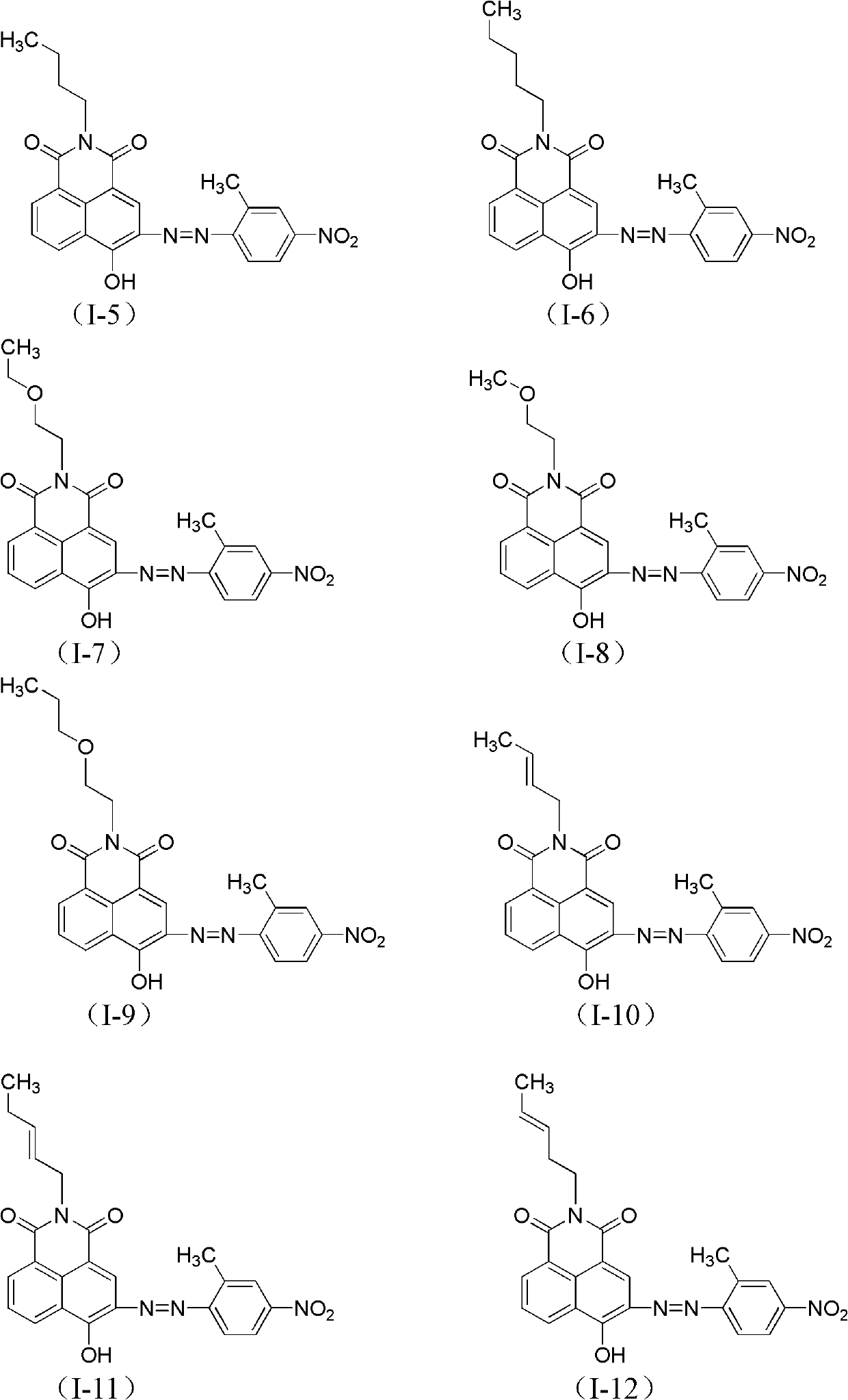
Azonaphthalene dicarboximide compound and composition, preparation and application thereof
A technology of azonaphthalimide and compound, which is applied in the field of disperse dyes, can solve the problems of only grade 4 fastness to washing and rubbing fastness, staining with other colors, etc., and achieve good color fixing rate and good Effect of dyeing property and excellent light fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) Chlorination reaction
[0045] Dissolve 20g of 1,8-naphthalene dicarboxylic anhydride (industrial product content is 99%, 0.1mol, molar weight 23.26g) in 200g sodium hypochlorite solution (available chlorine content is 10%), heat and reflux for 4 hours, cool, and use carbonic acid Sodium hydrogen was used to adjust the pH to 7.5±0.5, and the water was distilled off under reduced pressure, washed with 3×20 mL of water and dried to obtain 22.3 g of 4-chloro-1,8-naphthalene dicarboxylic anhydride (purity: 95.8%, 0.0918 mol), mol The yield was 91.8% (calculated as 1,8-naphthalene dicarboxylic anhydride, the same below).
[0046] (2) Imination reaction
[0047] 22.3g (purity is 95.8%, 0.0918mol) of 4-chloro-1,8-naphthalic anhydride prepared in step (1) and 9.5g methylamine aqueous solution (40% by mass, methylamine 0.122mol) are dissolved in In 220g of DMF (dimethylformamide), heat and reflux for 5 hours, cool, and remove the solvent DMF by distillation under reduced p...
Embodiment 2
[0054] (1) Chlorination reaction
[0055] The sodium hypochlorite charging capacity is changed into 240g, other conditions and operation are with embodiment 1, obtain 4-chloro-1,8-naphthalic anhydride 22.5g (purity is 96.2%, 0.093mol), molar yield 93.0% (with 1 , 8- naphthalene dicarboxylic anhydride).
[0056] (2) Imination reaction
[0057] The charging capacity of 40% methylamine is changed into 9.33g (methylamine 0.12mol), the charging capacity of DMF is changed into 170g, other conditions are the same as embodiment 1, obtain 4-chloro-1,8-naphthalimide 21.2g (content 96.1%, 0.0829mol), molar yield 89.1% (based on 4-chloro-1,8-naphthalene dicarboxylic anhydride).
[0058] (3) Hydrolysis reaction
[0059] The charging capacity of sodium hydroxide is changed into 4.2g (content 95%, 0.1mol), the charging capacity of methyl alcohol is changed into 128g, other conditions are the same as embodiment 1, obtain 4-hydroxyl-1,8-naphthalene dicarboxylic acid Amine 17.4g (purity 96....
Embodiment 3
[0063] (1) Chlorination reaction
[0064] The sodium hypochlorite charging capacity is changed into 220g, other conditions are the same as embodiment 1, obtain 4-chloro-1,8-naphthalene dicarboxylic anhydride 22.4g (purity is 96.0%, 0.0924mol), molar yield 92.4% (with 1, 8-naphthalic anhydride).
[0065] (2) Imination reaction
[0066] The charging capacity of methylamine is changed into 10.1g (0.13mol), the charging capacity of DMF is changed into 260g, other conditions are the same as embodiment 1, obtain 4-chloro-1,8-naphthalimide 22.1g (purity is 96.5%, 0.0868mol), and the molar yield is 93.9% (based on 4-chloro-1,8-naphthalene dicarboxylic anhydride).
[0067] (3) Hydrolysis reaction
[0068] The charging capacity of sodium hydroxide is changed into 4.7g (0.112mol), the charging capacity of methyl alcohol is changed into 214g, and other conditions are the same as embodiment 1, obtain 4-hydroxyl-1,8-naphthalimide 18.3g ( The purity is 96.9%, 0.0837mol), and the molar yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com