Economic, practical, and environment friendly method for preparing norathyriol
A mangiferin aglycone and reaction technology, applied in the direction of organic chemistry and the like, can solve the problems of difficult product separation, great environmental hazards, and high reagent toxicity, and achieve the effects of short process route, cheap raw materials, and few synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of mangiferin aglycone by the method of the present invention
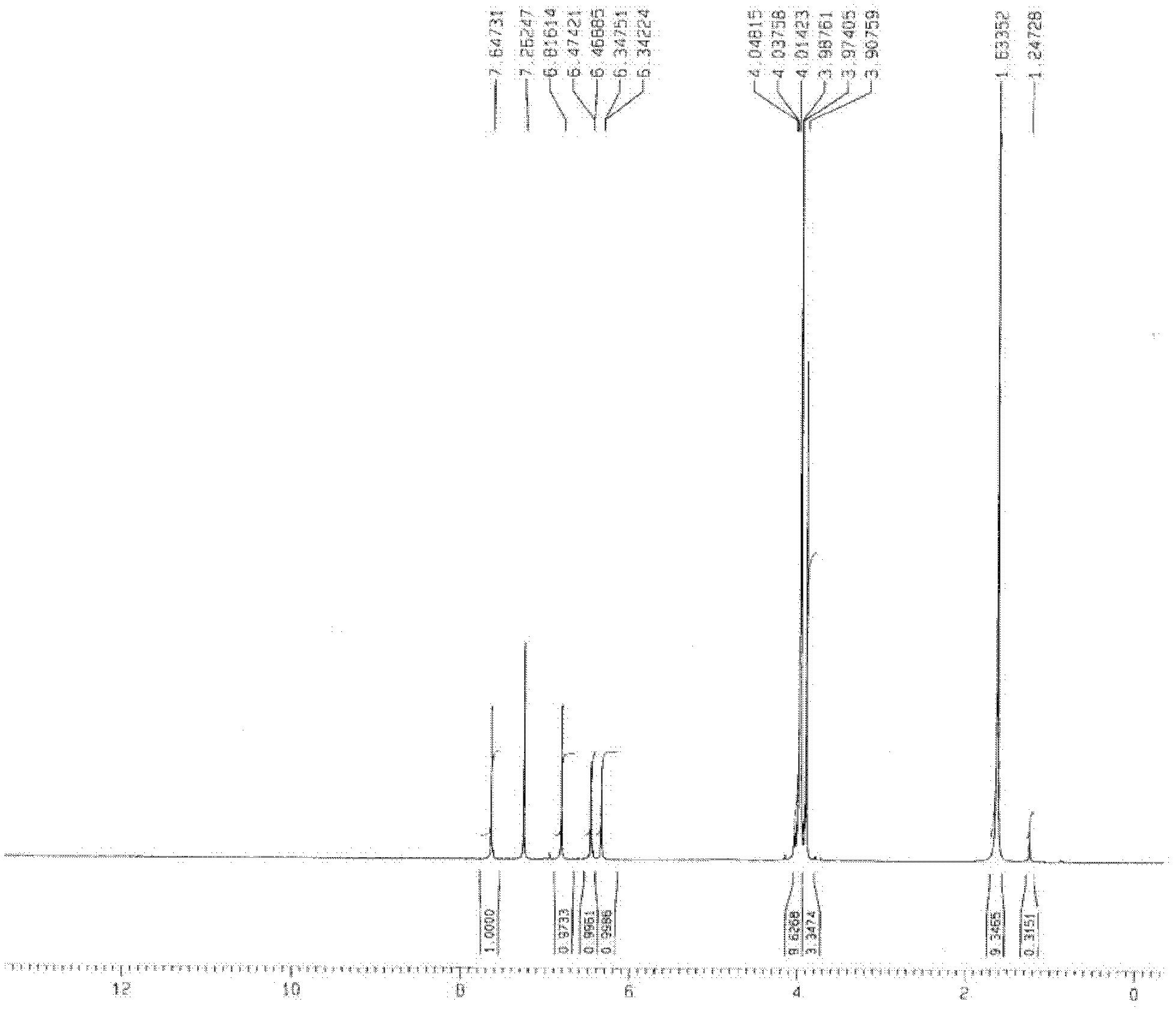
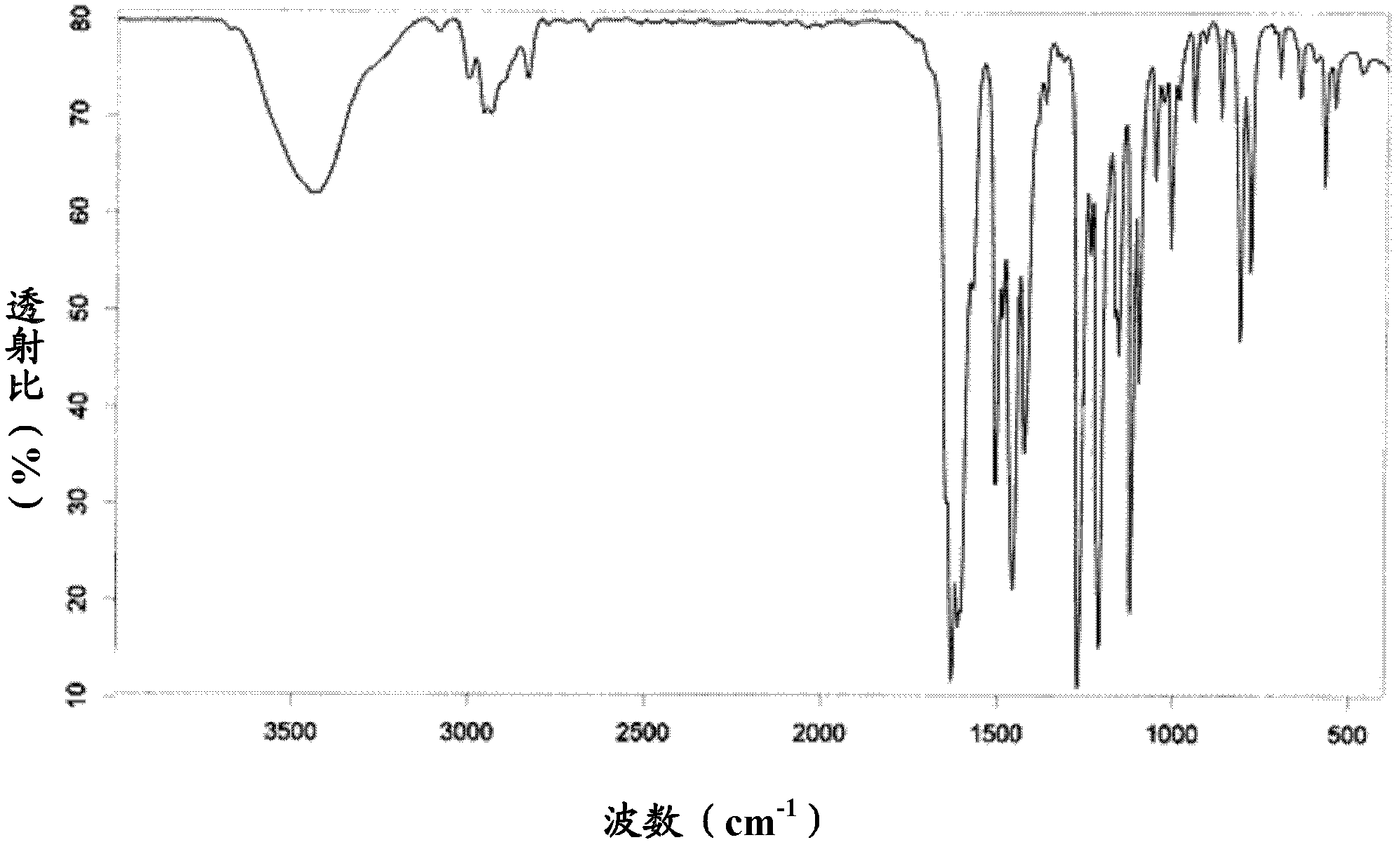
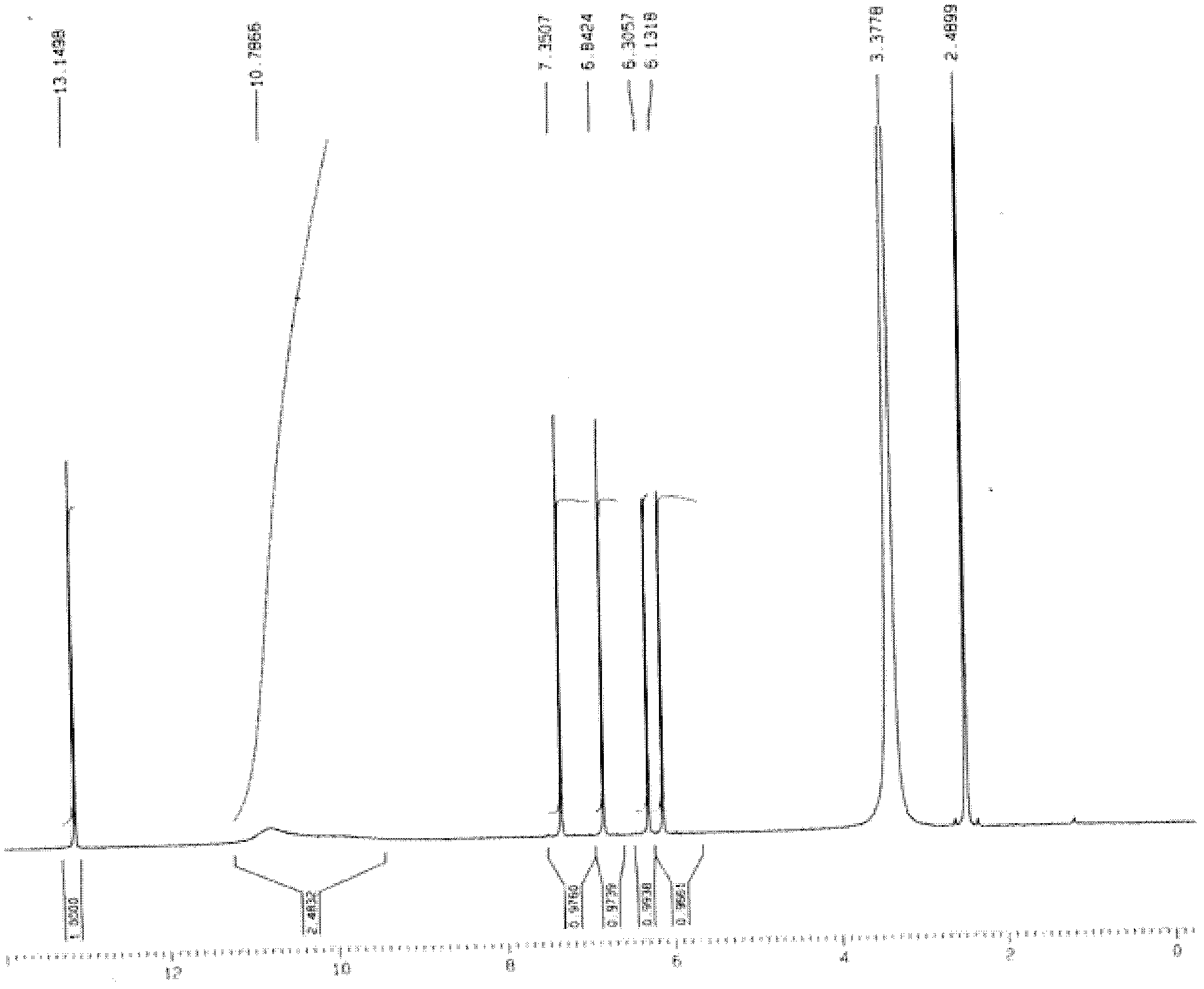
[0039] Weigh 26.1g (0.10mol) of 2-bromo-4,5-dimethoxybenzoic acid and put it into a 250mL reaction flask, add 20mL of 1,2-dichloroethane, 16.7g (0.14mol) of thionyl chloride , heated to reflux for 1 hour, added 20.4g of zinc chloride (0.15mol) and 18.5g (0.12mol) of 3,5-dimethoxyphenol, stirred, continued to heat at 75 ° C for 2.5 hours, steamed out 1 under reduced pressure, 2-dichloroethane solvent, add 80.0g of sodium hydroxide aqueous solution (about 0.20mol sodium hydroxide) with a concentration of 10%, continue to heat and reflux for 2 hours, cool down, a large amount of white solids are precipitated, filter, and the filtrate is washed with water to The water washing solution was neutral, drained and dried to obtain 28.4 g of white 1,3,6,7-tetramethoxyxanone with a yield of 90%. IR v max (KBr)cm -1 : 3442, 2961, 2939, 2836, 1627, 1507, 1457, 1425, 1271, 1211, 1158, 1122; 1 HN...
Embodiment 2
[0041] Example 2: Preparation of mangiferin aglycone by the method of the present invention
[0042] Weigh 104.4g (0.40mol) of 2-bromo-4,5-dimethoxybenzoic acid and put it into a 500mL reaction flask, add 100mL of 1,2-dichloroethane, 59.5g (0.50mol) of thionyl chloride , heated to reflux for 1 hour, added 54.4g (0.40mol) of zinc chloride and 65.0g (0.42mol) of 3,5-dimethoxyphenol, stirred, continued to heat at 60 ° C for 3 hours, steamed out 1 under reduced pressure, 2-dichloroethane solvent, add 120.0g of sodium hydroxide aqueous solution (about 0.60mol sodium hydroxide) with a concentration of 20%, continue to heat under reflux for 3 hours, cool down, a large amount of white solids are precipitated, filter, and the filtrate is washed with water to The water washing solution was neutral, drained and dried to obtain 113.0 g of white 1,3,6,7-tetramethoxyxanone with a yield of 89%. IR v max (KBr)cm -1 : 3442, 2961, 2939, 2836, 1627, 1507, 1457, 1425, 1271, 1211, 1158, 1122; ...
Embodiment 3
[0044] Example 3: Preparation of mangiferin aglycone by the method of the present invention
[0045] Weigh 52.2g (0.20mol) of 2-bromo-4,5-dimethoxybenzoic acid and put it into a 250mL reaction flask, add 40mL of 1,2-dichloroethane, 35.7g (0.30mol) of thionyl chloride , heated to reflux for 1 hour, added 32.6g (0.24mol) of zinc chloride and 30.8g (0.20mol) of 3,5-dimethoxyphenol, stirred, continued to heat at 40°C for 4 hours, steamed out 1 under reduced pressure, 2-dichloroethane solvent, add 80.0g of sodium hydroxide aqueous solution (about 0.60mol sodium hydroxide) with a concentration of 30%, continue to heat under reflux for 2.5 hours, cool down, and precipitate out a large amount of white solids, filter, and the filtrate is washed with water to The water washing solution was neutral, drained and dried to obtain 56.9 g of white 1,3,6,7-tetramethoxyxanone with a yield of 90%. IR v max (KBr)cm -1 : 3442, 2961, 2939, 2836, 1627, 1507, 1457, 1425, 1271, 1211, 1158, 1122; 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com