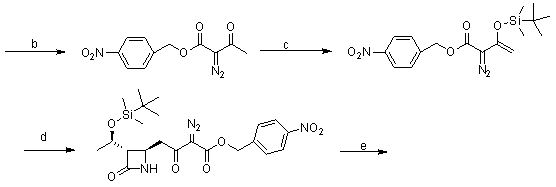
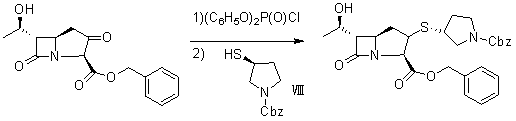Synthesis method of panipenem and intermediates thereof
A synthesis method and compound technology, which are applied in the field of synthesis of panipenem and its intermediates, can solve the problems of great harm to the environment and operators, high price, and unsuitable for scale-up production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0056] In order to make the technical means, creative features, work flow, and use methods of the present invention achieve the purpose and effect easily understood, the present invention will be further described below in conjunction with specific embodiments.
[0057] Synthesis of Compound II:
[0058] Add 232 grams (2.00mol) of methyl acetoacetate, 237.6 grams (2.20mol) of benzyl alcohol and 3000 milliliters of toluene into the reaction flask, and slowly add 45 grams (0.30mol) of boron trifluoride ether solution with a concentration of 46% under stirring. ), reflux reaction for 6 hours, after the reaction, the reaction solution was poured into ice water, washed with saturated aqueous sodium bicarbonate solution until neutral, the water layer was extracted with 1 liter of toluene, the toluene layers were combined, washed with water, anhydrous sodium sulfate Dry; filter, the filtrate is concentrated to dryness, and the residue is distilled off under reduced pressure to remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com