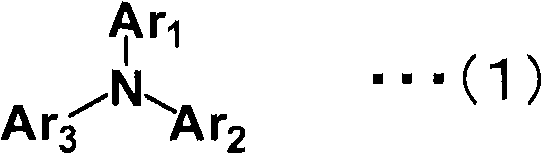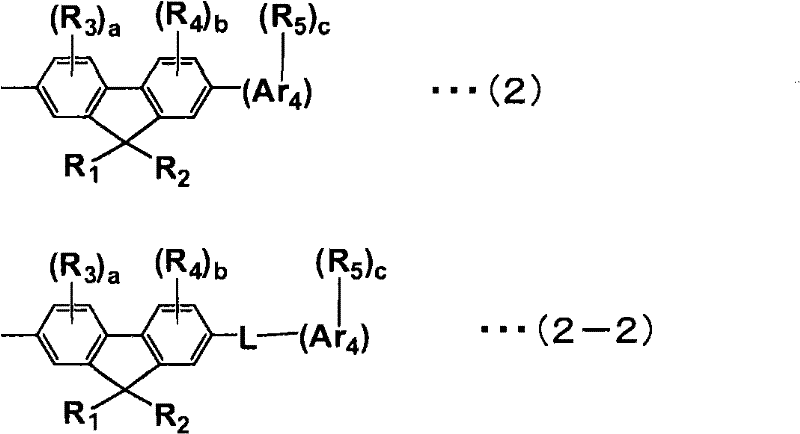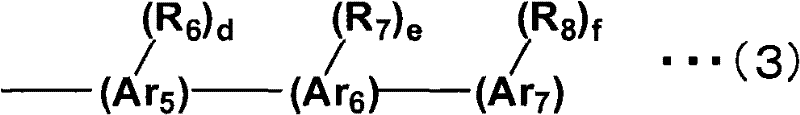Aromatic amine derivative and organic electroluminescent element using same
A technology of aromatic amines and derivatives, which is applied in the field of aromatic amine derivatives and organic electroluminescent elements using them, and can solve problems such as blocked outlets, crystallization, and lower yields of light-emitting elements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0167] Hereinafter, a production example of an organic electroluminescence element having a structure in which an anode / hole injection layer / light-emitting layer / electron injection layer / cathode are sequentially provided on a light-transmitting substrate is given. First, an anode is prepared by forming a thin film of an anode material on a suitable translucent substrate by vapor deposition or sputtering to a film thickness of 1 μm or less (preferably within a range of 10 to 200 nm). Next, a hole injection layer was provided on the anode. The formation of the hole injection layer can be carried out by methods such as vacuum evaporation method, spin coating method, casting method, LB method, etc. as described above, but it is preferable to use formed by vacuum evaporation. When the hole injection layer is formed by vacuum evaporation, the evaporation conditions vary depending on the compound used (the material of the hole injection layer), the crystal structure and the recombin...
Embodiment
[0171] Hereinafter, the present invention will be described in more detail based on synthesis examples and examples.
[0172] The structural formulas of Intermediates 1 to 14 produced in Synthesis Examples 1 to 14 are shown below.
[0173]
Synthetic example 1
[0174] Synthesis Example 1 (Synthesis of Intermediate 1)
[0175] Under argon flow, add 47g of 4-bromobiphenyl, 23g of iodine, 9.4g of periodic acid dihydrate, 42mL of water, 360mL of acetic acid, and 11mL of sulfuric acid into a 1000mL three-necked flask, and stir at 65°C for 30 minutes. The reaction was carried out at 90° C. for 6 hours. The reactant was poured into ice water and filtered. After washing with water, methanol was used to obtain 67 g of a white powder. By FD-MS analysis, relative to C 12 h 8BrI=359, the main peaks of m / z=358 and 360 were obtained, which were identified as Intermediate 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| visible light transmittance | aaaaa | aaaaa |
| electron work function | aaaaa | aaaaa |
| electron work function | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com