Testing method for related substances of drug combination containing tegafur, gimeracil and oteracil potassium
A technology of oteracil potassium and gimeracil, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as difficult detection methods, and achieve scientific impurity calculation methods, shortened separation time, and good separation effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
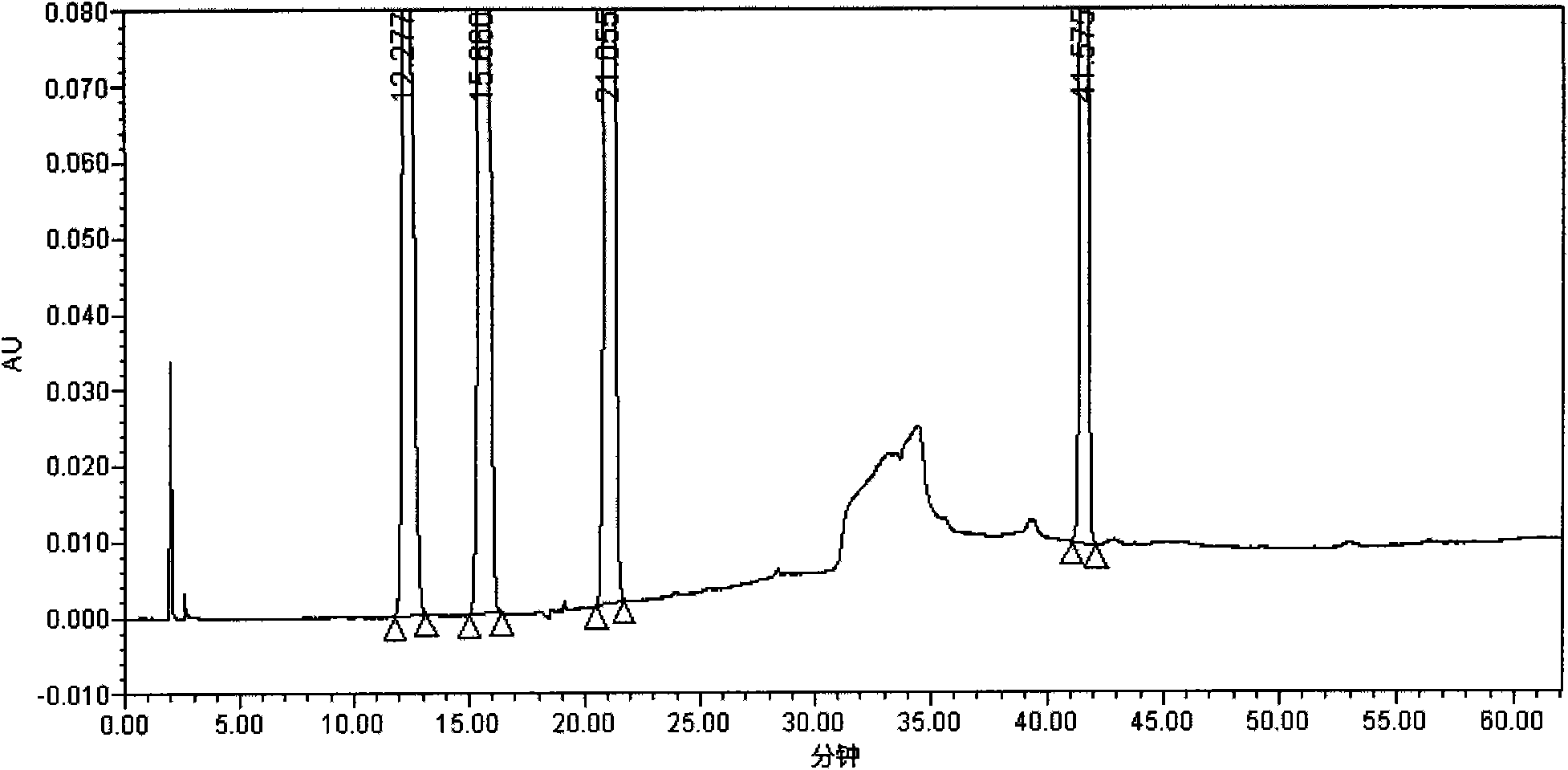
[0034] Instruments and conditions: Waters 2695 liquid chromatography system, PDA detector, chromatographic column: Inertsil ODS-3 (4.6mm×150mm, 5μm); detection wavelength: 220mn; column temperature: 35°C; g of potassium dihydrogen phosphate is dissolved in water and diluted to 1800ml, add 4ml of 10% tetrabutylammonium hydroxide, mix well, adjust the pH value to 3.2 with phosphoric acid)-methanol (94:6) as mobile phase A, methanol-acetonitrile (7 : 1) is mobile phase B, and carries out linear gradient elution according to the following table.
[0035]
[0036] experiment procedure:
[0037] 1. Peak positioning test: Take 24.5 mg of oteracil potassium reference substance, 25.1 mg of tegafur reference substance, 7.3 mg of gimeracil reference substance, and 7.2 mg of impurity A reference substance, weigh them accurately, and put them in the same 100ml measuring bottle. Add 30% acetonitrile aqueous solution (pH11.2) to dissolve and dilute to the mark, shake well, measure accord...
Embodiment 2
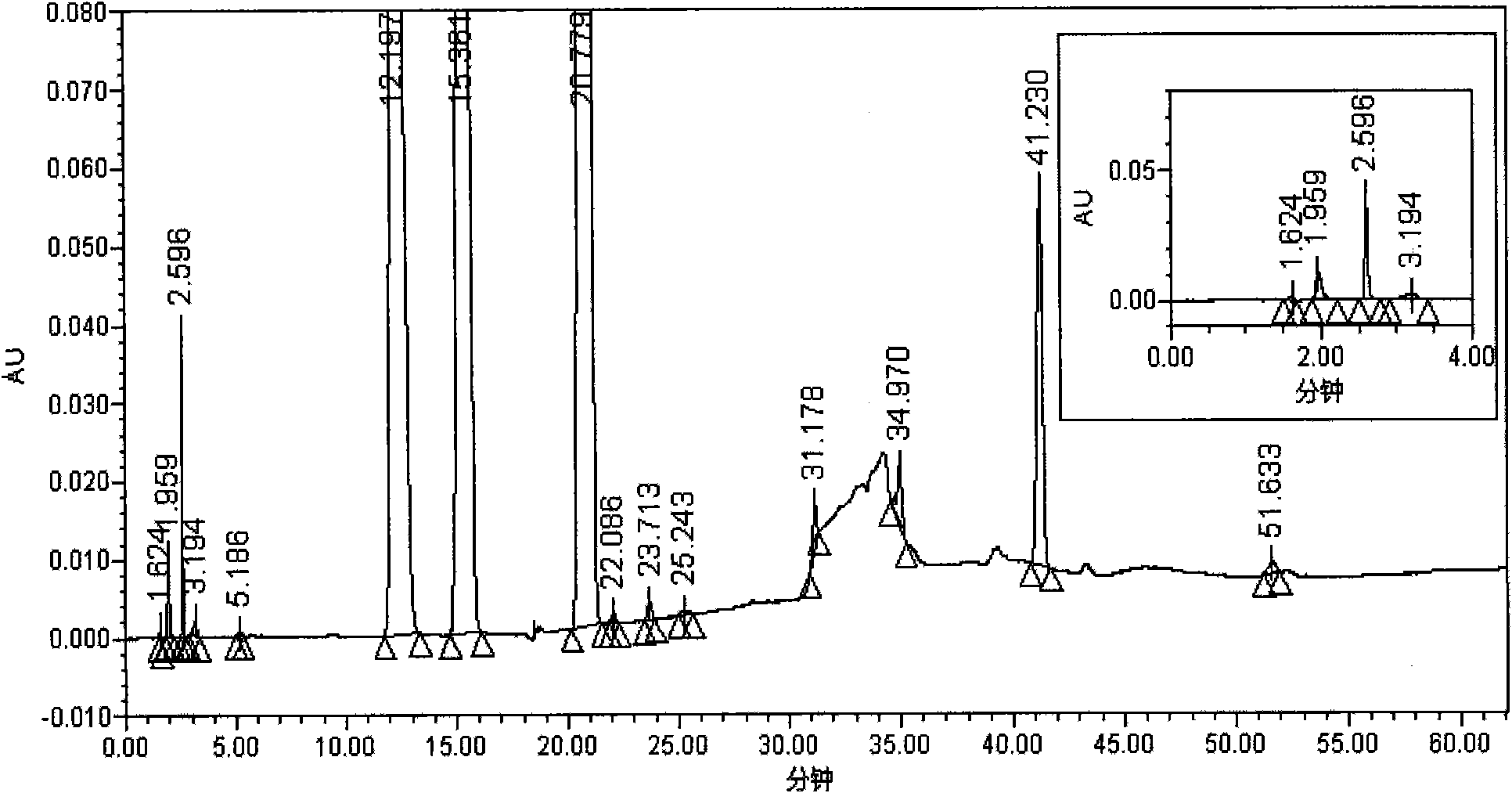
[0051] Instruments and conditions: Agilent 1200 liquid chromatography system, VWD detector, chromatographic column: Inertsil ODS-3 (4.6mm×150mm, 5μm); detection wavelength: 220nm; column temperature: 35°C; g of potassium dihydrogen phosphate is dissolved in water and diluted to 1800ml, add 4ml of 10% tetrabutylammonium hydroxide, mix well, adjust the pH value to 3.2 with phosphoric acid)-methanol (94:6) as mobile phase A, methanol-acetonitrile (7 : 1) is mobile phase B, and carries out linear gradient elution according to the following table.
[0052]
[0053] Test procedure: Take appropriate amounts of Tegafur, Gimeracil, Oteracil Potassium, and Impurity A reference substance respectively, and accurately weigh them to make concentrations of 1.00 μg / ml, 0.29 μg / ml, 0.98 μg / ml, and 0.29 μg The mixed solution of the reference substance per ml was diluted according to the stepwise dilution method, and the quantification limits and detection limit. The limit of quantification...
Embodiment 3
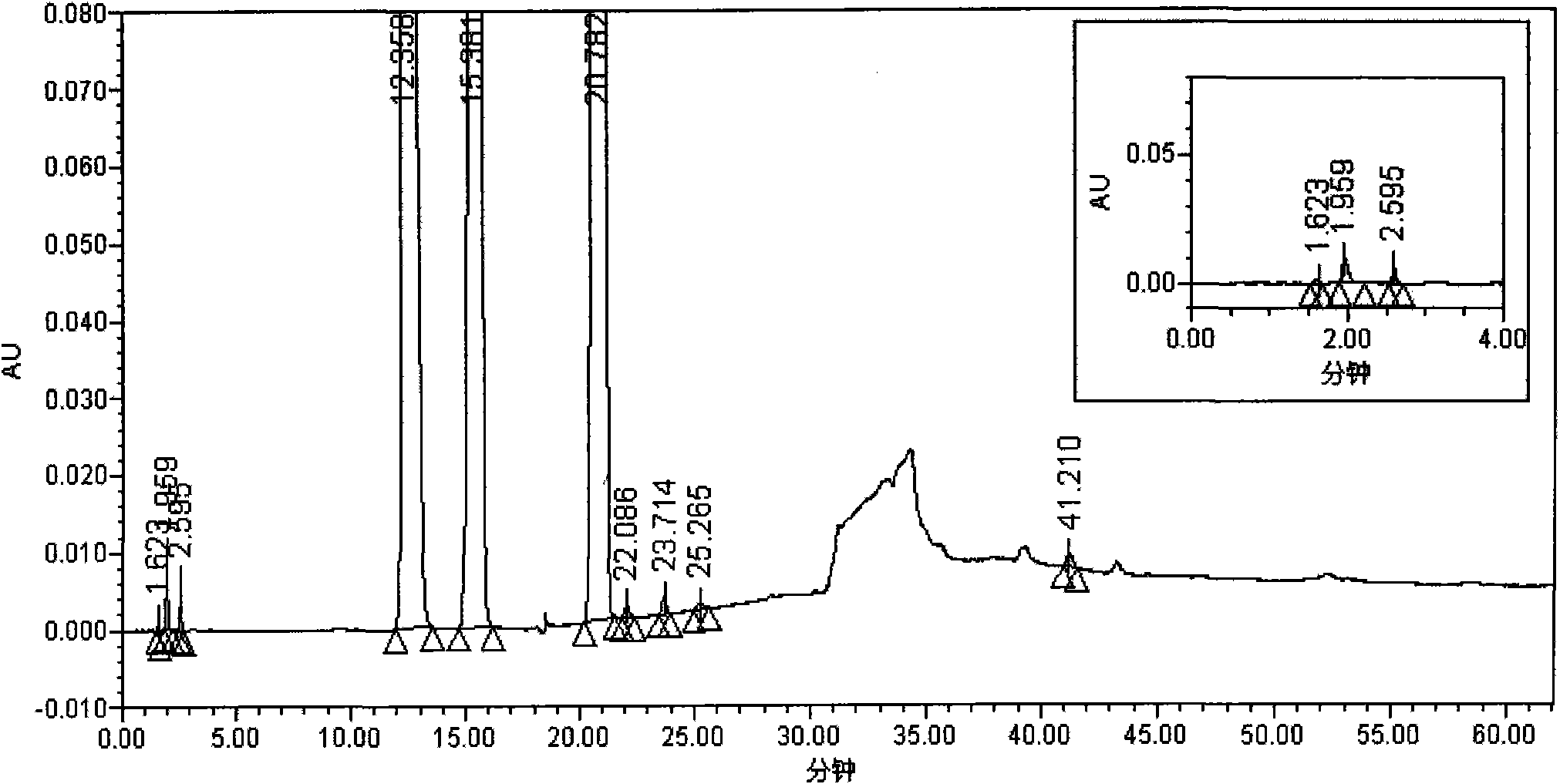
[0056] Instruments and conditions: Waters 2695 liquid chromatography system, PDA detector, chromatographic column: Welch Materials C18 (4.6mm×250mm, 5μm); detection wavelength: 220nm; column temperature: 35°C; phosphate buffer (take 1.3g Dissolve potassium dihydrogen phosphate in water and dilute to 1800ml, add 4ml of 10% tetrabutylammonium hydroxide, mix well, adjust the pH value to 3.2 with phosphoric acid) as mobile phase A, methanol-acetonitrile (1:1) as mobile phase B, Carry out linear gradient elution according to the table below.
[0057]
[0058]
[0059] Test procedure: Take 104.6mg of the accelerated 12-month sample of S-1 capsule, put it in a 25ml measuring bottle, add 30% acetonitrile aqueous solution (pH 11.2) to dissolve and dilute to the mark, shake well, filter, and measure according to the law.
[0060] As can be seen from the test results: the detection method of the present invention can separate the main peaks of the S-Gio Capsules and its impurity pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com