Preparation method of inorganic phosphatic crystal material
A technology of inorganic phosphate and crystalline materials, applied in molecular sieve and alkali exchange phosphate, molecular sieve characteristic aluminum phosphate, etc., can solve the problems of high crystallization temperature, high energy consumption, long time, etc., to increase lithium ion content, improve The effect of the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The above-mentioned inorganic phosphate crystal material adopts a hydrothermal synthesis method, and the preparation method comprises the following steps:
[0039] a) Mix the aluminum source, sodium phosphate and / or lithium phosphate, fluoride, and deionized water in a molar ratio of 3-5:6-10:6-10:20-100, and fully stir to form an initial condensation glue;
[0040] b) Use sodium hydroxide solution and / or lithium hydroxide solution to adjust the pH value of the initial gel obtained in step a) to 8-9; preferably the pH value is 8-8.5, and the recommended pH value is greater than 8.5 and less than or equal to 9, The preferred pH value is greater than or equal to 8.2 and less than 8.5.
[0041] c) Crystallize the gel obtained in step b) under hydrothermal conditions to obtain the inorganic phosphate crystal material; the crystallization temperature is 120-160° C., and the crystallization time is 12-90 hours; preferably, the crystallization temperature The temperature is ...
Embodiment 1
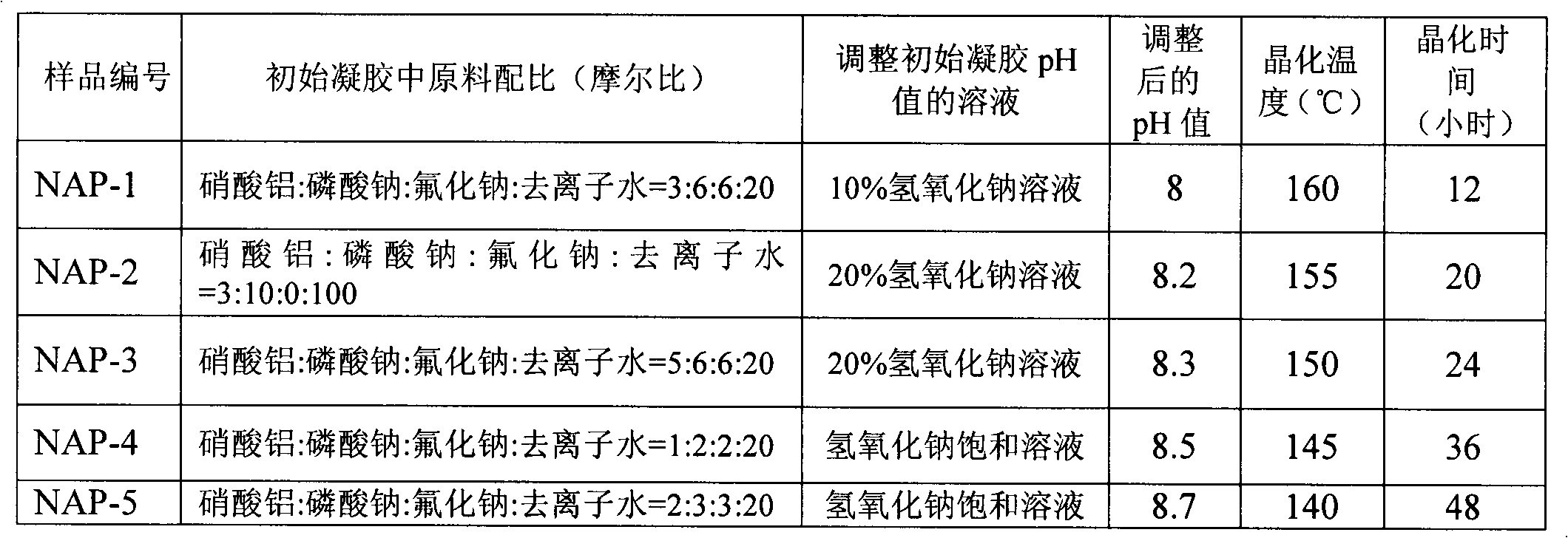
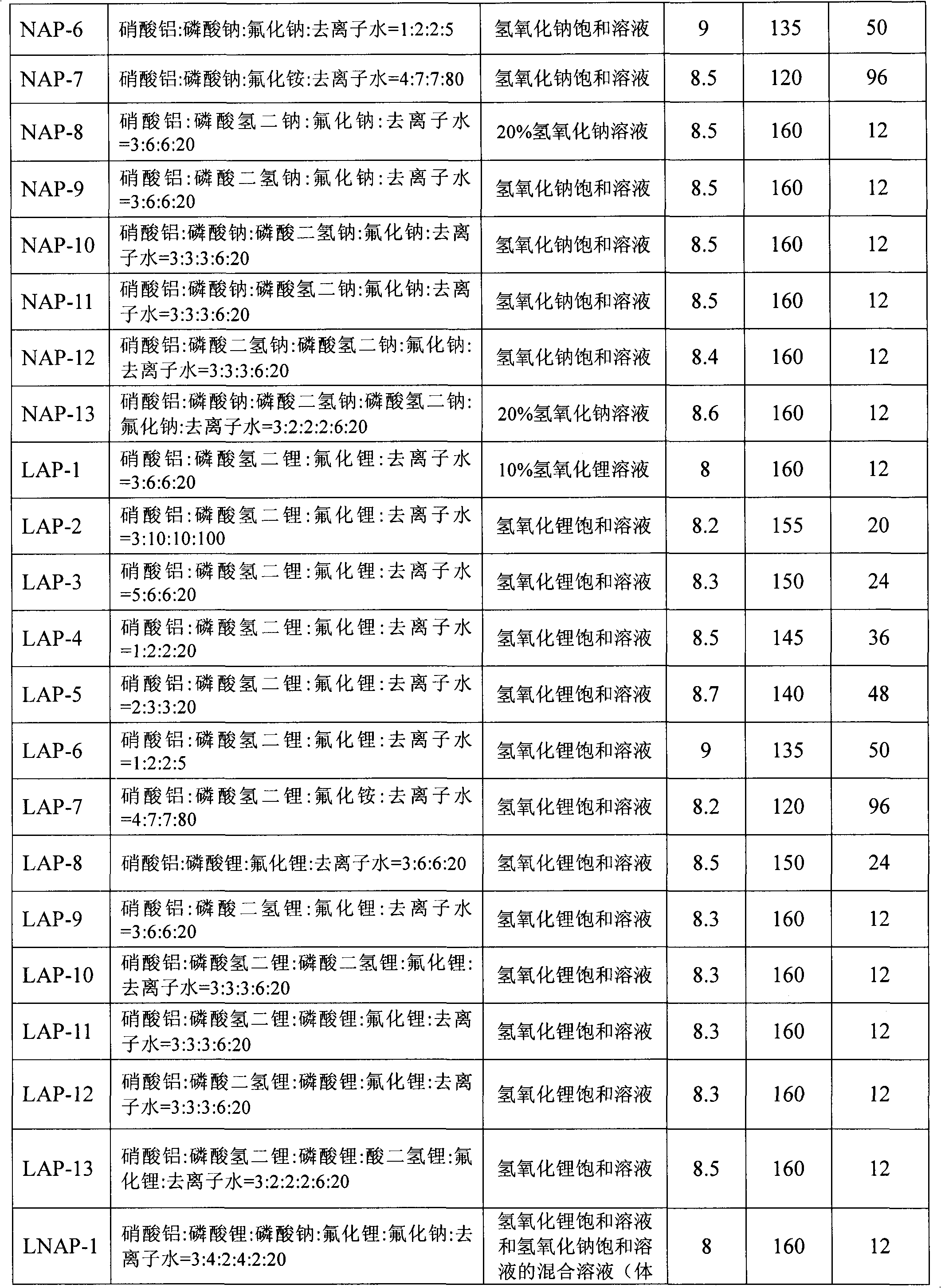
[0059] Mix the aluminum source, sodium phosphate and / or lithium phosphate, fluoride, and deionized water in a certain proportion, stir at room temperature for 0.5 hours to form an initial gel, add dropwise sodium hydroxide solution and / or lithium hydroxide solution, adjust its pH value to a certain value in 8-9, and stir for 1 hour. The obtained gel was transferred into a stainless steel kettle equipped with polytetrafluoroethylene lining, placed in an oven for crystallization at a certain temperature for a period of time, and the obtained product was washed three times with deionized water to obtain a white crystal sample. The corresponding relationship between the sample number and the ratio of raw materials in the initial gel, the sodium hydroxide solution and / or lithium hydroxide solution used to adjust the pH value of the initial gel, the adjusted pH value, the crystallization temperature, and the crystallization time is as follows: Table 1 shows.
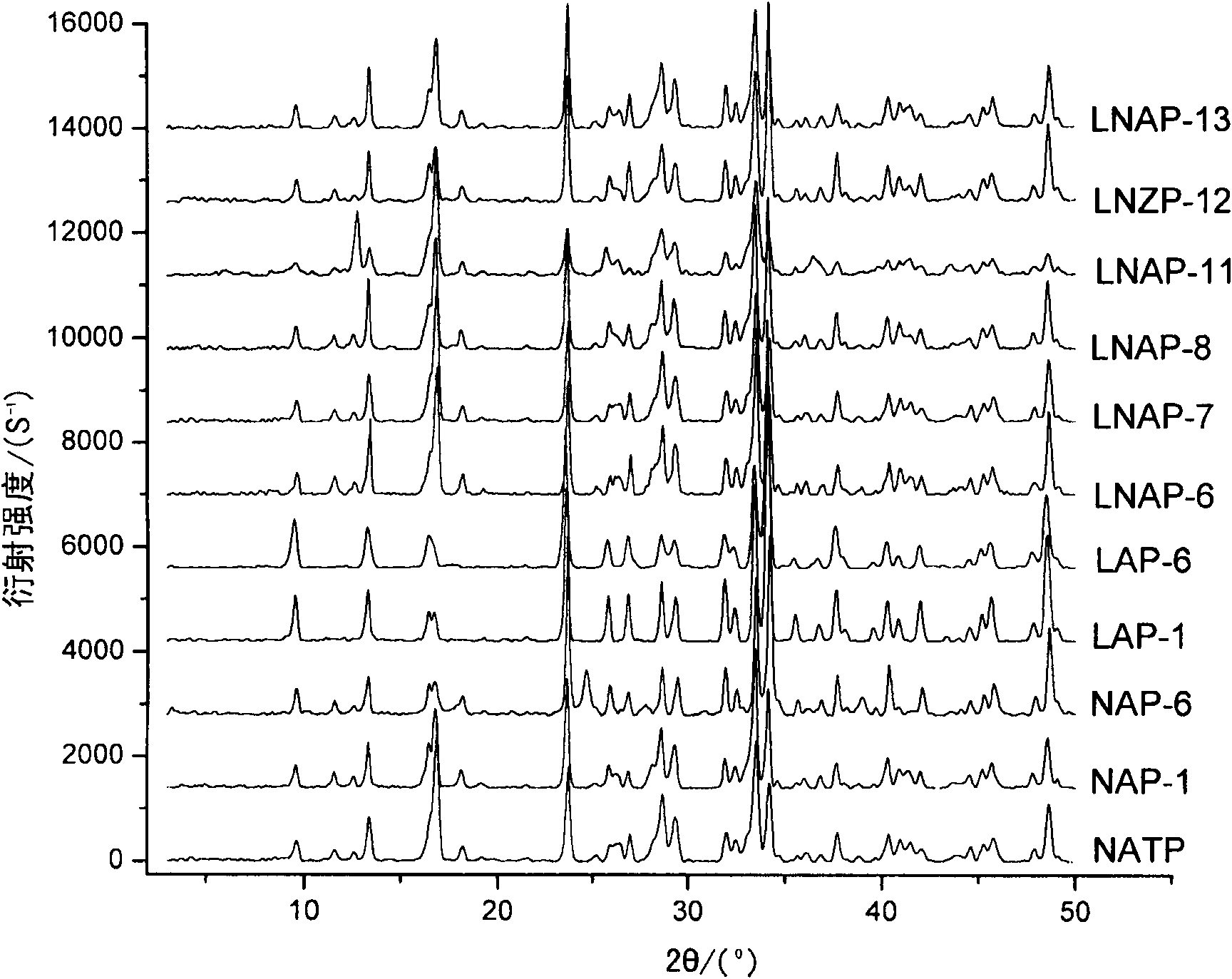
[0060] Table 1: Corr...
Embodiment 2
[0066] Elemental analysis of the samples NAP-1, LAP-1, LNAP-6, LNAP-7, LNAP-8, LNAP-11, LNAP-12, and LNAP-13 prepared in Example 1, elements with an atomic number greater than 8 Adopt Philips Magix X-ray fluorescence spectrometer to measure the elemental composition of sample, the content of Li in the sample adopts IRIS Advantage full-frequency plasma direct-reading spectrometer (ICP) to measure, and the results are shown in Table 2 and figure 1 .
[0067] Table 2: Analysis results of sample composition elements
[0068] Sample serial number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com