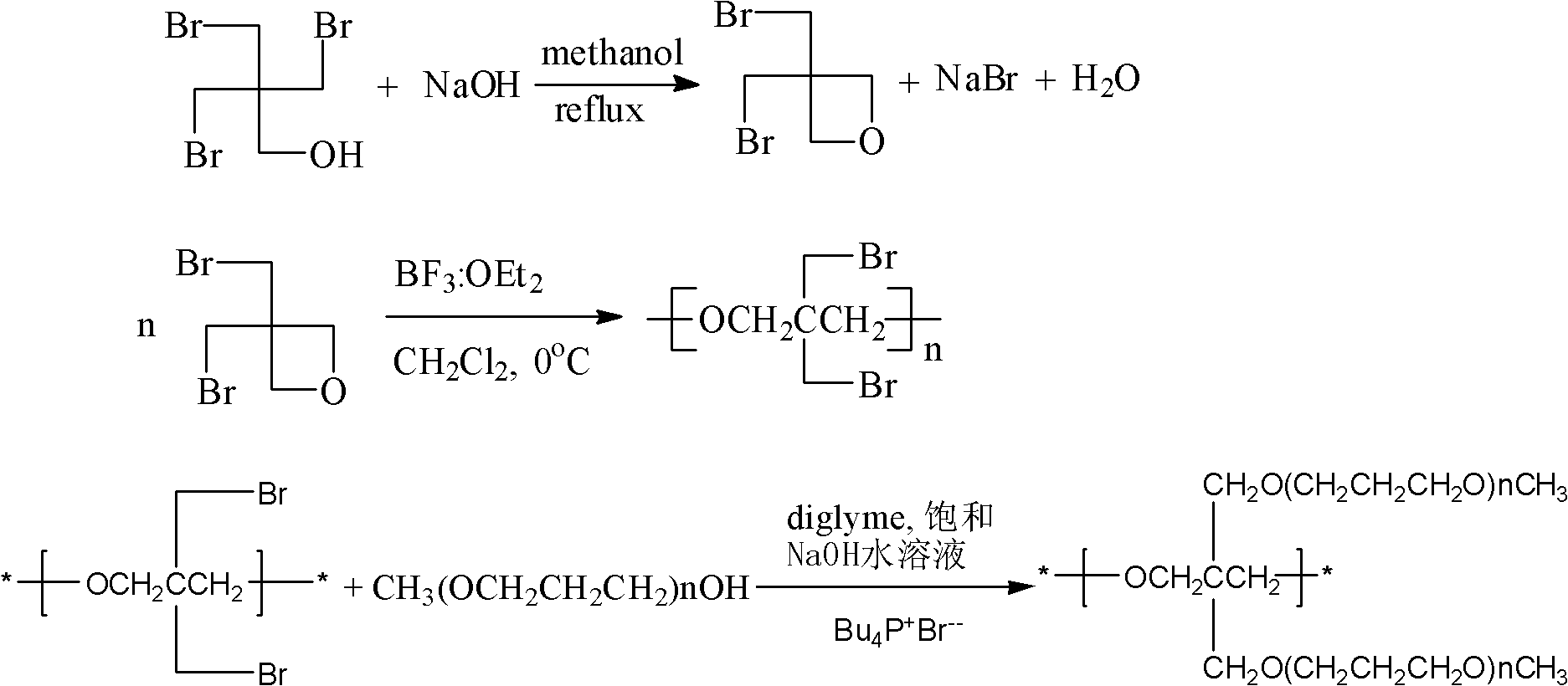Comb-shaped polymer, comb-shaped polymer electrolyte material and preparation method of the comb-shaped polymer electrolyte material
A comb-like polymer and electrolyte material technology, applied in the field of polymers, to achieve good mechanical properties, enhance mobility, and increase capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
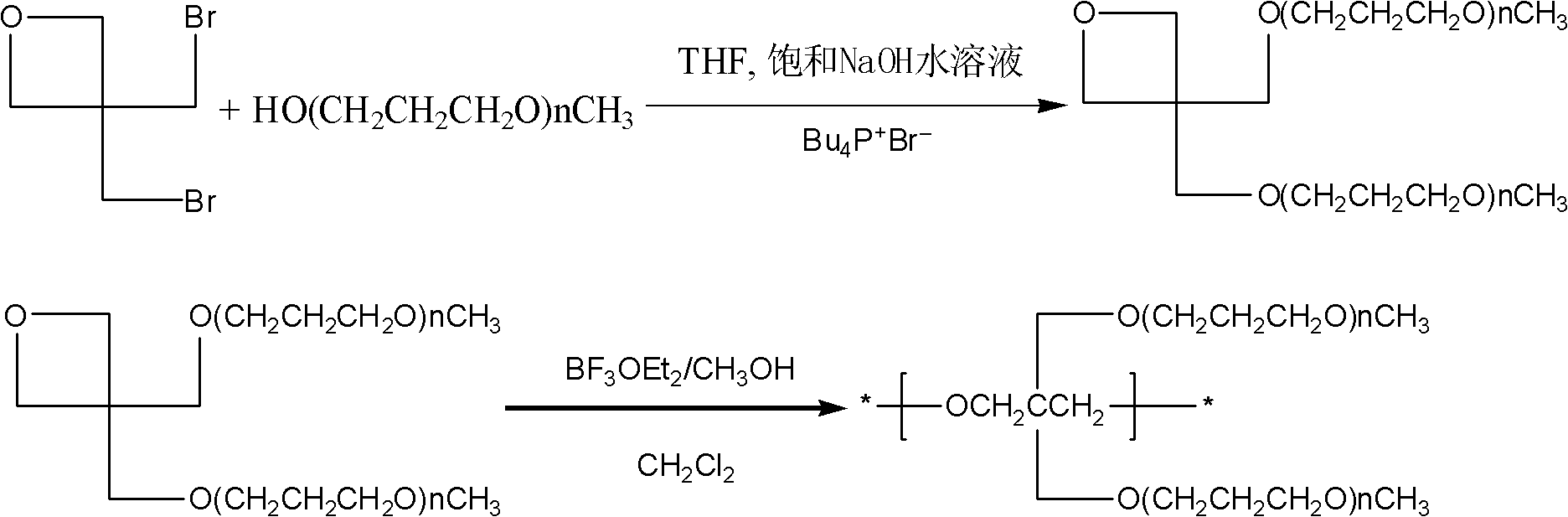
[0059] Mix 32.5g of tribromoneopentyl alcohol, 4.44g of sodium hydroxide, 40mL of methanol and 10mL of water in a round-bottomed flask, stir and react at 60°C under nitrogen protection, and react for 1 hour, filter to remove the generated NaBr, and spin The solvent was removed, and the product was distilled under reduced pressure (5 mm Hg) at 80° C. to obtain 3,3-bis(bromomethyl)oxetane (21.5 g, yield 88%) as a colorless oil.
[0060] Add 18g of 3,3-bis(bromomethyl)oxetane and 30mL of dichloromethane solvent into a dry three-necked flask, and mix well at 0°C. Quickly inject 15uL boron trifluoride diethyl ether initiator. The reaction proceeded vigorously, and poly[3,3-bis(bromomethyl)oxetane] was obtained as a white solid within a few minutes. The obtained polymer was washed successively with chloroform and aqueous sodium bicarbonate solution, and then vacuum-dried to obtain 17.4 g of the product, with a yield of 97%.
[0061] 13g poly[3,3-bis(bromomethyl)oxetane] homopolyme...
Embodiment 2
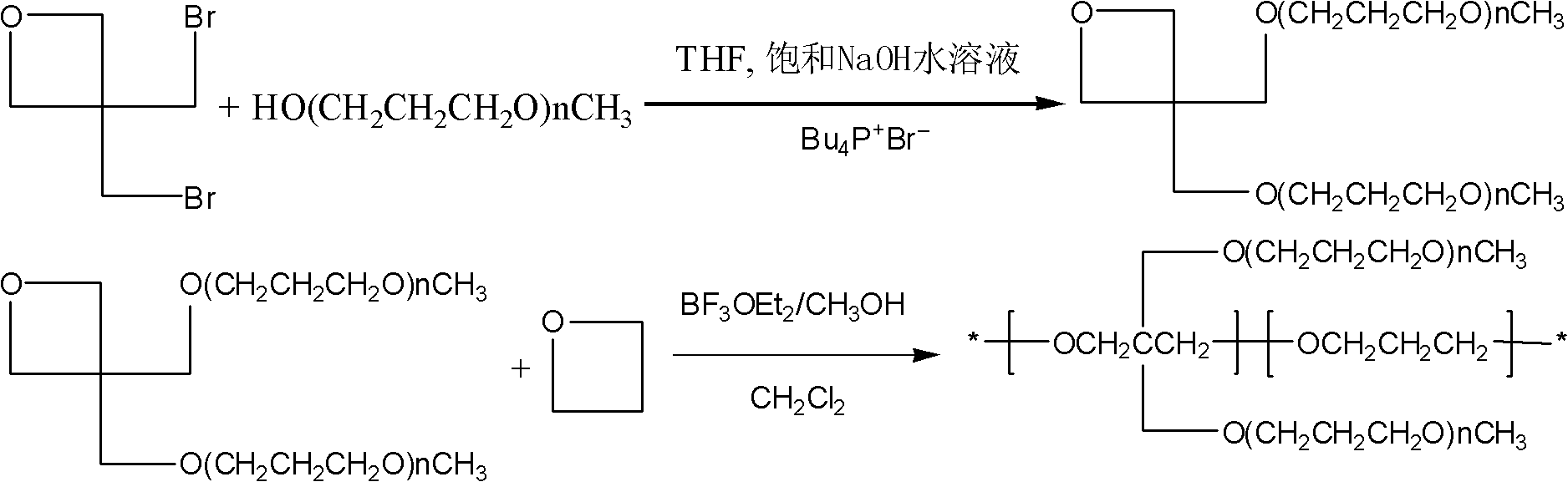
[0066] Add 30uL boron trifluoride diethyl ether and 2mL dichloromethane into a dry three-necked flask, and mix well at 0°C. Then 3.42 g of oxetane, 6 g of 3,3-bis(bromomethyl)butylene oxide prepared in Example 1, and 10 mL of dichloromethane were slowly added (addition rate: 0.5 mL / min). After reacting for 1 hour, the obtained random copolymer was neutralized with 0.02 g of sodium bicarbonate aqueous solution, the polymer was precipitated with petroleum ether, and the dissolution-precipitation was repeated twice, the solvent was rotary evaporated, and the product was vacuum-dried at 70 ° C for 24 hours to obtain 7.2 g The product yield was 76.4%. The molar ratio of oxetane to 3,3-bis(bromomethyl)oxetane in the random copolymer was 3.4:1.
[0067] With 4.41g poly[oxetane-3,3-bis(bromomethyl)oxetane] copolymer, 1g tetrabutylphosphine bromide, 7.92g methyl polyoxypropylene alcohol (4 repetitions unit), 10 mL of diethylene glycol dimethyl ether were mixed in a beaker and slowly a...
Embodiment 3
[0072] Add 30uL boron trifluoride diethyl ether and 2mL dichloromethane into a dry three-necked flask, and mix well at 0°C. Then 3.42 g of oxetane, 6 g of 3,3-bis(bromomethyl)oxetane prepared in Example 1, and 10 mL of dichloromethane were slowly added (addition rate: 0.5 mL / min). After reacting for 1 hour, the obtained random copolymer was neutralized with 0.02 g of sodium bicarbonate aqueous solution, the polymer was precipitated with petroleum ether, and the dissolution-precipitation was repeated twice, the solvent was rotary evaporated, and the product was vacuum-dried at 70 ° C for 24 hours to obtain 7.2 g The product yield was 76.4%. The molar ratio of oxetane to 3,3-bis(bromomethyl)oxetane in the random copolymer was 3.4:1.
[0073] 4.41g poly[oxetane-3,3-bis(bromomethyl)oxetane] copolymer, 1g tetrabutylphosphine bromide, 21.84g methyl polyoxypropylene alcohol (12 repeated unit), 20 mL of diethylene glycol dimethyl ether were mixed in a beaker and slowly added to a thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com