Preparation method of shape controllable monodisperse barium sulfate crystal particles
A monodisperse, barium sulfate technology, applied in the direction of calcium/strontium/barium sulfate, etc., can solve the problems of high energy consumption and high equipment investment, and achieve the effects of energy saving, uniform particle size and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
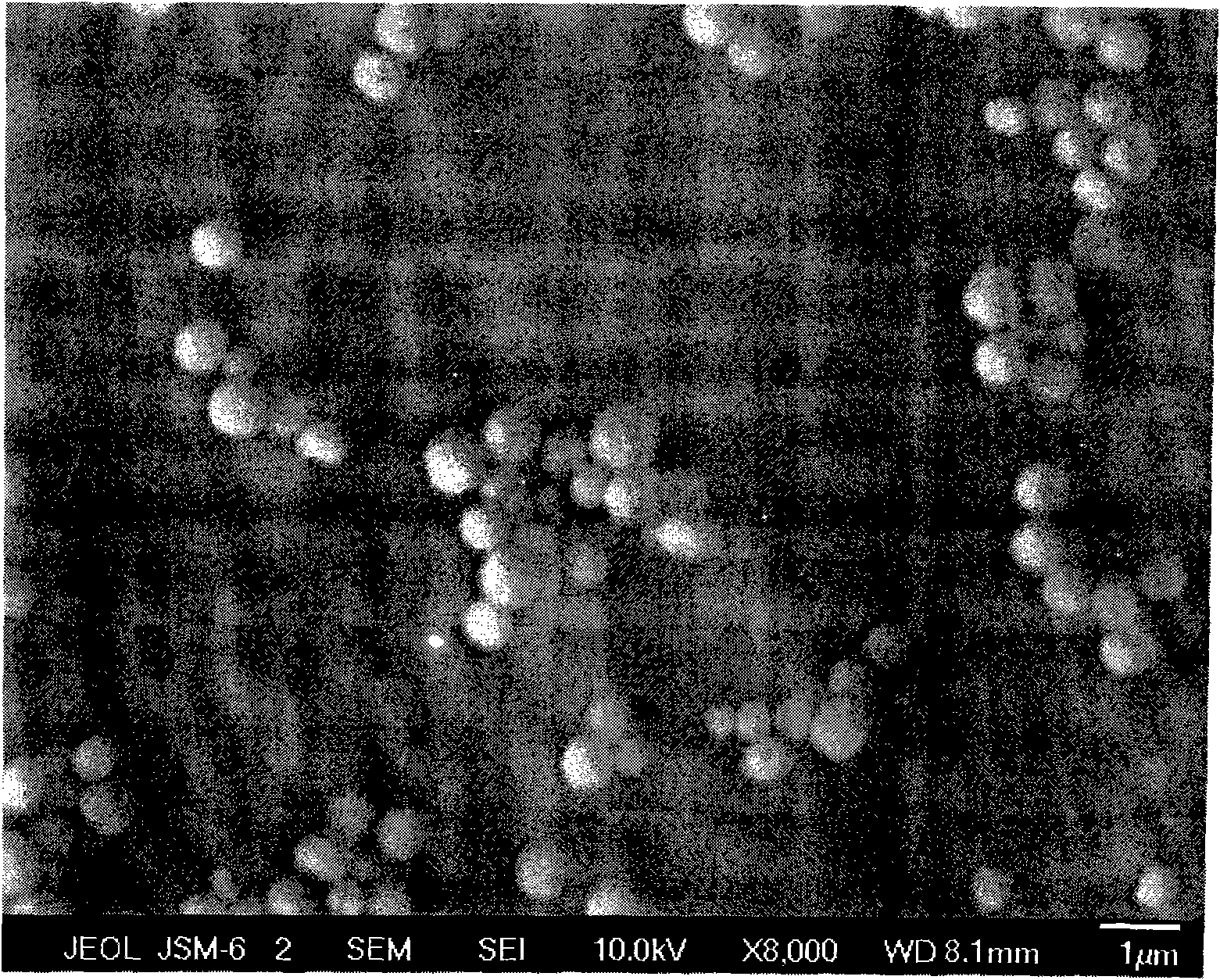
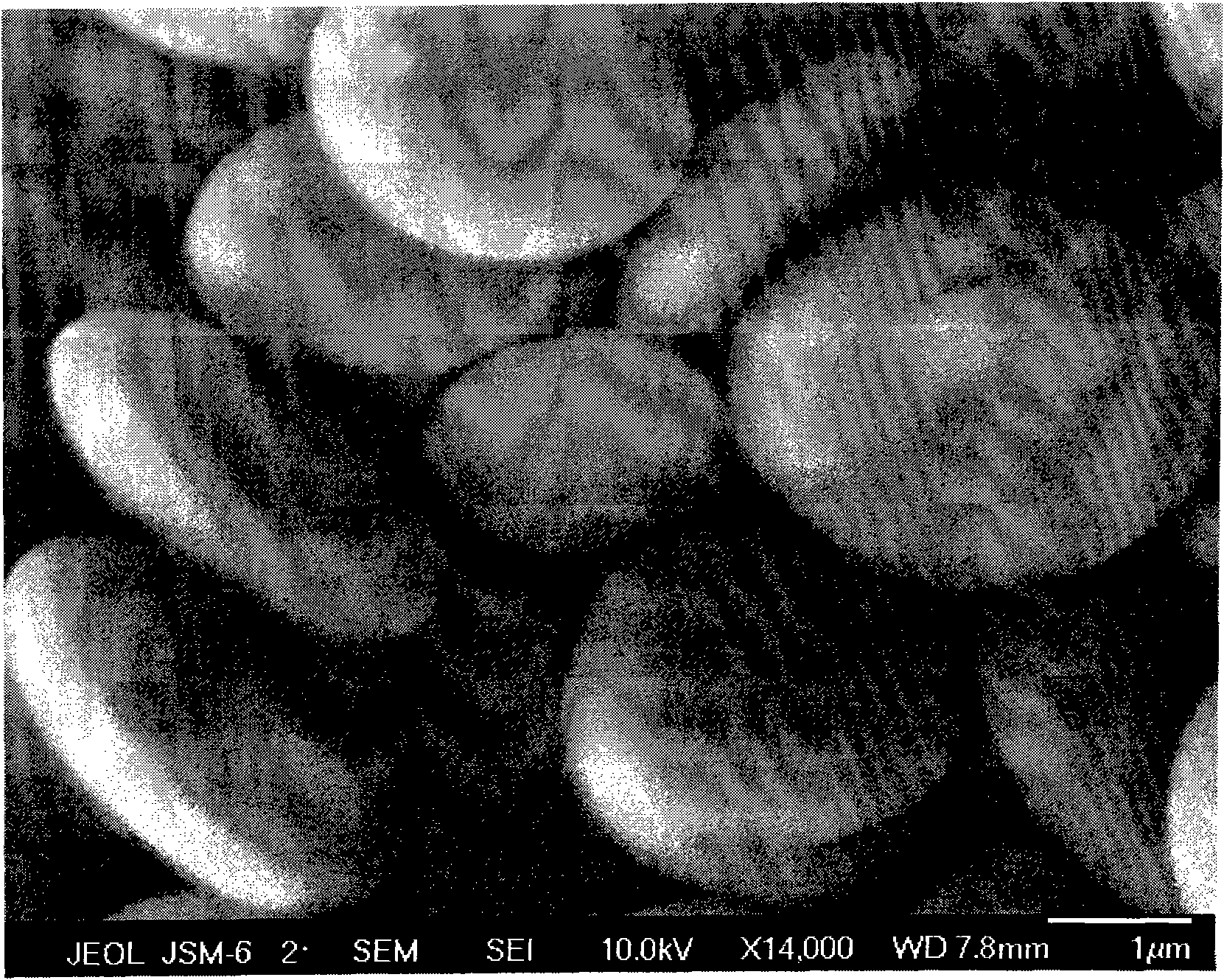
[0029]After the 1L glass beaker was cleaned, the preparation concentration was 0.001g / L sulfonated polyaspartic acid derivative (this sulfonated polyaspartic acid derivative refers to the literature (Macromolecular Materials Science and Engineering, 2008, 24, 44-50) is prepared by reacting taurine with polysuccinimide (m / n=40 / 100) aqueous solution, and the pH value of the aqueous solution of sulfonated polyaspartic acid derivatives is adjusted to 1 with hydrochloric acid under stirring, so that The same flow rate of 3ml / min injected 2mol / L barium acetate aqueous solution and 2mol / L ammonium sulfate aqueous solution into the aqueous solution of sulfonated polyaspartic acid derivatives at the same time; Stop injecting barium acetate aqueous solution and ammonium sulfate aqueous solution after the turbidity, cover the beaker with a layer of film to cover dust, leave it to age at room temperature for 5 hours, separate and dry, and obtain spherical barium sulfate crystal particles w...
Embodiment 2
[0032] After cleaning the 1L glass beaker, prepare hydroxylated polyaspartic acid (hydroxylamine and polyaspartic acid) with a concentration of 2g / L, at 80°C, in the form of P 2 o 5 Prepare the dehydrating agent through amidation reaction, m / n=10 / 100) aqueous solution, adjust the pH value of the aqueous solution of hydroxylated polyaspartic acid to 7 with aqueous sodium hydroxide solution under stirring, and simultaneously use the same flow rate of 0.2ml / min Inject 0.1mol / L barium nitrate solution and 0.1mol / L sodium sulfate solution into the hydroxylated polyaspartic acid aqueous solution; stop injecting barium nitrate aqueous solution and sulfuric acid after the hydroxylated polyaspartic acid aqueous solution produces obvious turbidity Sodium aqueous solution, covered with a layer of film to cover dust on the beaker, static aging at room temperature for 10 hours, separated and dried to obtain spherical barium sulfate crystal particles with a particle size of about 0.5 μm.
...
Embodiment 3
[0035] After cleaning the 1L glass beaker, prepare a mixed aqueous solution containing 0.1g / L polyaspartic acid and 0.001g / L 2-aminoethanol, adjust the pH value of the above mixed aqueous solution to 13 with potassium hydroxide under stirring, and use 3ml At the same flow rate per minute, inject 10mol / L barium chloride aqueous solution and 10mol / L potassium sulfate aqueous solution into the above-mentioned mixed aqueous solution at the same time; stop injecting barium chloride aqueous solution and potassium sulfate aqueous solution after the above-mentioned mixed aqueous solution is obviously turbid. Cover with a layer of film to cover dust, aging at room temperature for 24 hours, separate and dry to obtain disc-shaped barium sulfate crystal particles with a particle size of about 1.5 μm.
[0036] The SEM photo of the prepared barium sulfate crystal particles is shown in Figure 1c .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com