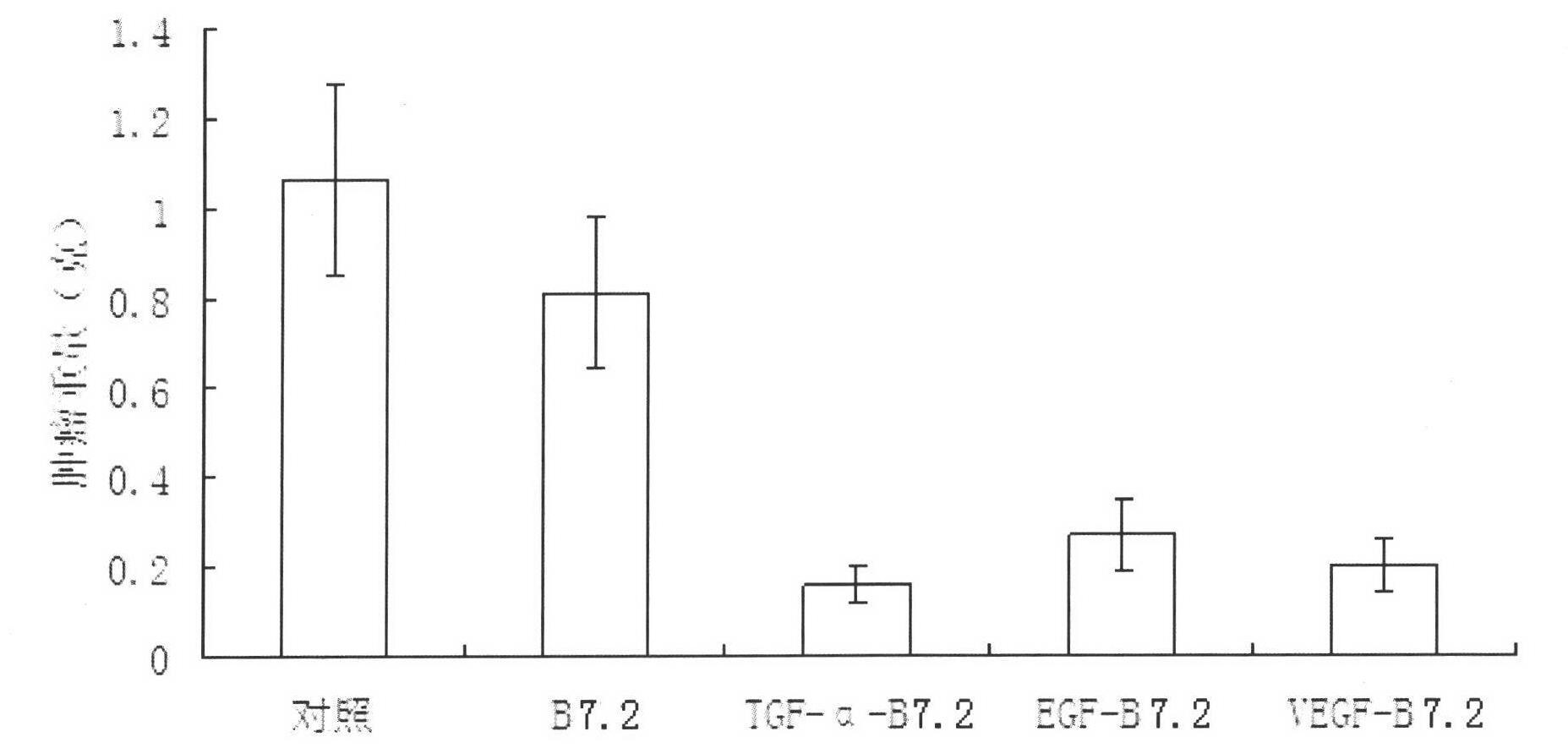
Fusion protein capable of inducing and activating cancer-targeted T cells, preparation method and use thereof
A fusion protein and cancer cell technology, applied in the field of biomedicine, to achieve the effect of inhibiting tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Construction of B7.2 (CD86) expression vector
[0032] According to the information (NM_175862 and L25259) of the B7.2 gene (also known as CD86) of human origin in the GenBank database, TAKARA was commissioned to synthesize a nucleic acid sequence fragment including a connecting peptide and a B7.2 gene (222 extracellular parts of the code Amino acid) and a few bases of HindIII in front of the whole fragment and XhoI restriction enzyme cutting point in the back, insert this synthesized nucleic acid fragment into T vector and carry out DNA sequencing identification, and then use HindIII and XhoI by double restriction method After treatment, this fragment was inserted into the pET22b plasmid, thus producing the expression vector pET22b-B7.2, which can express the B7.2 protein. The sequence listing (SEQ ID NO.2) is only the B7.2 protein, and the previous connecting peptide can be found in another sequence listing (SEQ ID NO.14).
Embodiment 2
[0033] Example 2 Construction of TGF-α-B7.2 expression vector
[0034] According to the TGF-α gene information (NM_003236) of human origin in the GenBank database, TAKARA was commissioned to synthesize a nucleic acid sequence fragment including the TGF-α gene and several restriction endonuclease sites BamHI and EcoRI in front of TGF-α. bases, the end of the fragment contains the restriction endonuclease sites of SalI and HindIII. The synthesized nucleic acid fragment was inserted into the T vector and identified by DNA sequencing, and then treated with BamHI and HindIII by double enzyme digestion, and then inserted into the pET22b-B7.2 plasmid, thus producing the expression vector pET22b - TGF-α-B7.2, capable of expressing TGF-α-B7.2 fusion protein (see SEQ ID NO.4 in the sequence listing).
Embodiment 3
[0035] Example 3 Construction of EGF-B7.2 expression vector
[0036] According to the information of the EGF gene (NM_001963 and NM_001178130) of the human origin of GenBank database, entrust TAKARA company to synthesize a nucleic acid sequence fragment and comprise EGF gene and several bases of restriction endonuclease site BamHI and EcoRI in front of EGF again, in The rear of the fragment contains restriction endonuclease sites for SalI and HindIII. The synthesized nucleic acid fragment was inserted into the T vector and identified by DNA sequencing, and then treated with BamHI and HindIII by double enzyme digestion, and then inserted into the pET22b-B7.2 plasmid, thus producing the expression vector pET22b - EGF-B7.2, capable of expressing EGF-B7.2 fusion protein (see SEQ ID NO.6 in the sequence listing).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com