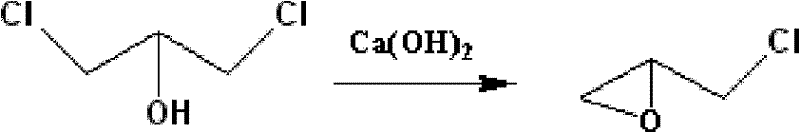Method for separating oil-phase dichloropropanol from mixed aqueous solution of dichloropropanol and chlorine hydride
A technology of dichloropropanol and mixed solution, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of hydroxyl compounds, etc., can solve problems such as increased equipment investment, difficulty in decanting, increased cost, etc., and can increase the amount of three wastes treated , Separation operation is simple, the effect of occupying less land
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for separating dichloropropanol oil phase from dichloropropanol hydrogen chloride mixed aqueous solution, comprising the steps:
[0035] (1) the dichloropropanol hydrogen chloride mixed aqueous solution that produces after the completion of the glycerol chlorination reaction of 10kg is put into the reactor (dichloropropanol hydrogen chloride mixed aqueous solution comprises the water of 53% weight, the 1,3 dichloropropanol of 33% weight Chloropropanol and 14% weight hydrogen chloride), the calcium chloride of 1.3kg is joined in the reactor, mixes and obtains the mixed solution containing calcium chloride;
[0036] (2) Heat the mixed solution containing calcium chloride to 130°C and stir for 5 hours. Hydrogen chloride, water and a small amount of dichloropropanol escape continuously in the reactor, and the oil phase and water phase of dichloropropanol are decanted and analyzed. layer, the water phase containing calcium chloride is sent to the drying section for ...
Embodiment 2
[0038] A method for separating dichloropropanol oil phase from dichloropropanol hydrogen chloride mixed aqueous solution, comprising the steps:
[0039] (1) put the dichloropropanol hydrogen chloride mixed aqueous solution that ferric trichloride and chloropropene method reaction finishes to produce in the reactor and mix to obtain the mixed solution that contains ferric chloride; After concentration, ferric chloride and dichlorohydrin The weight ratio of the mixed aqueous solution of chloropropanol and hydrogen chloride is 1:99; (after testing: the mixed aqueous solution of dichloropropanol and hydrogen chloride includes 73% by weight of water, 15% by weight of 1,3 dichloropropanol and 12% by weight of hydrogen chloride)
[0040] (2) Heat the mixed solution containing ferric chloride to 200° C., and stir for 8 hours under full vacuum to separate the dichloropropanol oil phase from the mixed solution.
Embodiment 3
[0042] A method for separating dichloropropanol oil phase from dichloropropanol hydrogen chloride mixed aqueous solution, comprising the steps:
[0043](1) Potassium bromide and propylene acetate method dichloropropanol hydrogen chloride mixed aqueous solution are put into the reactor and mixed to obtain a mixed solution containing potassium bromide; the weight ratio of potassium bromide and dichloropropanol hydrogen chloride mixed aqueous solution is 60 : 40; (through testing: dichloropropanol hydrogen chloride mixed aqueous solution comprises the water of 57% by weight, the 1 of 30% by weight, the hydrogen chloride of 3 dichloropropanol and 13% by weight)
[0044] (2) The mixed solution containing potassium bromide was mechanically stirred for 10 h under the conditions of 20° C. and 100 KPa gauge pressure, so that the dichloropropanol oil phase was separated from the mixed solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com