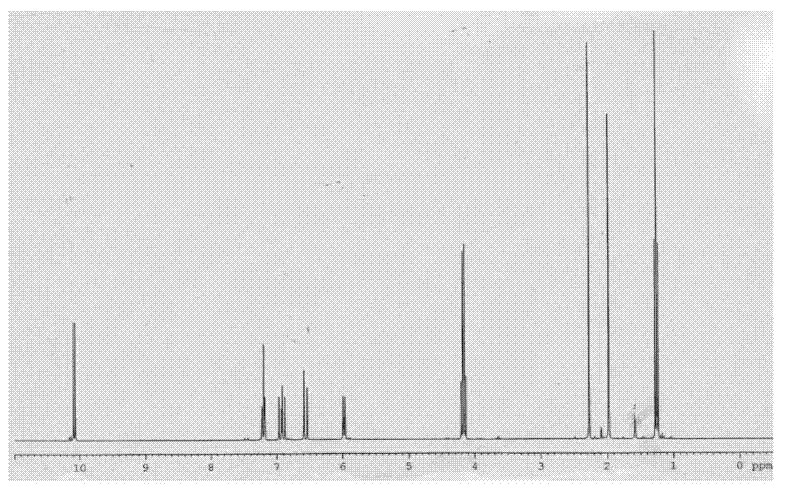
Method for synthesizing n-decanal ester
The technology of a decaaldehyde ester and a synthetic method is applied in the directions of chemical instruments and methods, preparation of carboxylic acid esters, preparation of organic compounds, etc., and can solve problems such as new routes and methods for synthesis without reports, high price, and no sales, etc., Achieve the effect of good industrialization prospects, simple industrialization operation, and little technical difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
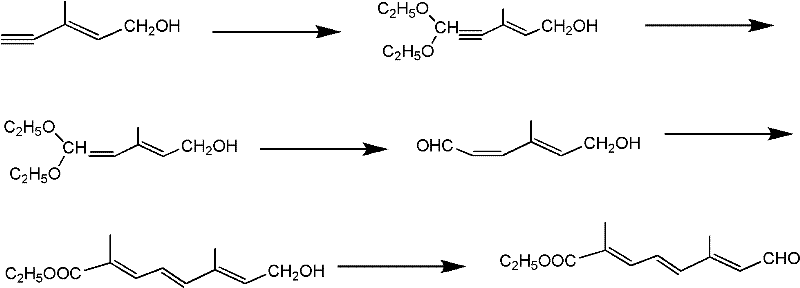
[0035] A kind of synthetic method of ten carbon aldehyde ester, comprises the following steps:
[0036] 1) Compound (E-4-acetoxy-2-methyl-2-butene-1-aldehyde) represented by structural formula (1) and trimethyl orthoformate or triethyl orthoformate at room temperature, acidic Catalyst reacts 10-15 hour under the p-toluenesulfonic acid effect, adds alkali after reaction and carries out hydrolysis, obtains the acetal product as shown in structural formula (2),
[0037]
[0038] Among them, R 1 It is methyl or ethyl, and the molar ratio of the compound shown in structural formula (1), trimethyl orthoformate or triethyl orthoformate, and base is 1: 0.8-1.5: 0.6-1.2;
[0039] 2) The acetal product with the structural formula obtained in the previous step as shown in (2) and the compound vinyl ether with the structural formula as shown in (3) are mixed in an organic solvent according to a molar ratio of 1: 0.8-1.2, at room temperature, a catalyst (ZnBr 2 or FeCl 3 or ZnCl 2 ...
Embodiment 1
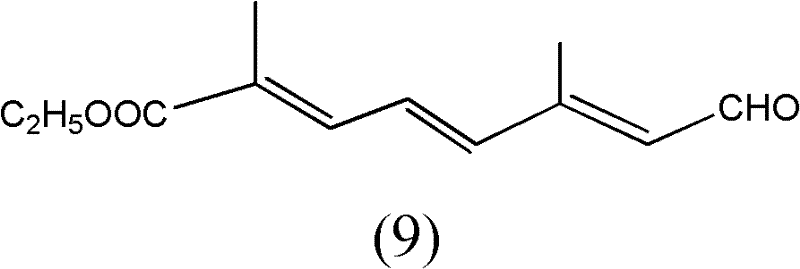
[0053] The preparation of the compound shown in structural formula as (9)
[0054]
[0055] Get 10 grams of the compound shown in structural formula (1), add catalytic amount of p-toluenesulfonic acid, add 10 grams of trimethyl orthoformate, react at room temperature for 10 hours, add 2 grams of hydrogen after the reaction finishes Sodium oxide, stirred and reacted for 6 hours, then added 200 milliliters of water, extracted three times with dichloromethane, 50 milliliters each time, combined the organic phases, backwashed the organic layer with 5% saline, 50 milliliters each time, dried with potassium carbonate, Filter, evaporate the solvent to dryness, and distill under reduced pressure to obtain 9 grams of the reaction product, the GC content is 98%, and the reaction equation is shown as follows:
[0056]
Embodiment 2
[0058] The preparation of the compound shown in structural formula as (10)
[0059]
[0060] Get 10 grams of the compound shown in structural formula (1), add catalytic amount of p-toluenesulfonic acid, add 11 grams of triethyl orthoformate, react at room temperature for 15 hours, add 2 grams of hydrogen after the reaction finishes Sodium oxide, stirred and reacted for 8 hours, then added 200 milliliters of water, extracted three times with dichloromethane, 50 milliliters each, combined the organic phases, backwashed the organic layer with 5% saline, 50 milliliters each, dried with potassium carbonate, Filter, evaporate the solvent to dryness, and distill under reduced pressure to obtain the reaction product of 8.5 grams, GC content 98%, and reaction equation is shown as follows:
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com