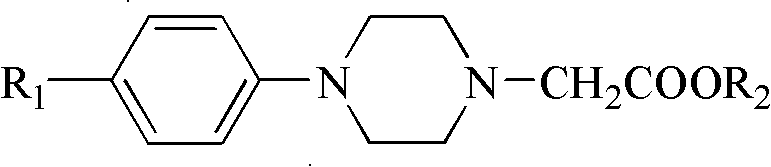
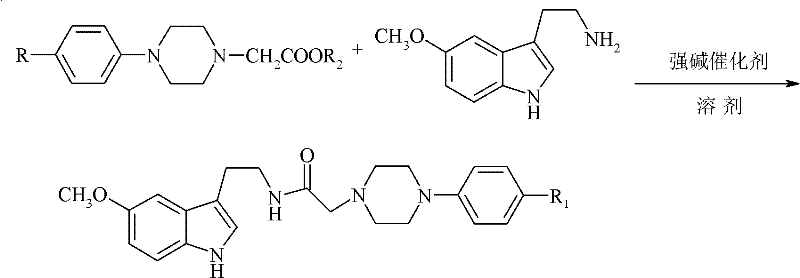
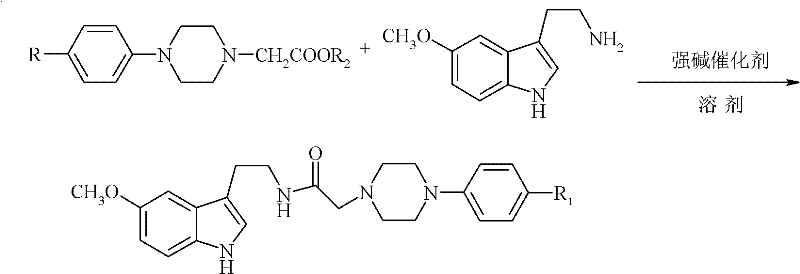
Method for preparing N-(5-methoxy-1H-indole-3-ethyl)-4-subtituted phenylpiperazine-1-acetamide
A technology of indoleethyl and phenylpiperazine, which is applied in the field of preparation of N--4-substituted phenylpiperazine-1-acetamide, can solve the problems of complicated operation, difficult solvent recovery, high toxicity and the like, and achieves the Safe and simple process operation, stable product quality and reduced toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In the reactor with low temperature and constant temperature reaction bath, stirring, thermometer, and reflux condenser, add 100ml ether, 23.7g (0.11mol) N-(4-methylphenyl)piperazine-1-acetic acid methyl ester, 1.4g sodium methoxide, control the reaction temperature 0-5 ℃, stir to obtain N-(4-methylphenyl)piperazine-1-acetic acid methyl ether mixture, add dropwise 20.9g (0.11mol) 5 - 5-methoxytryptamine ethyl ether solution composed of methoxytryptamine and 100ml ether, after reacting at the same temperature for 2 hours, slowly raise the temperature to 30°C, continue the reaction for 4 hours, and post-process the obtained reaction solution.
[0027] Add 10wt% dilute hydrochloric acid to wash until the pH value of the reaction solution is 5.6, wash the reaction solution twice with 20ml and 60ml of saturated sodium chloride solution after liquid separation, separate the liquid, dry with anhydrous sodium sulfate, filter, and recover by distillation under reduced pressure d...
Embodiment 2
[0029] In the reactor with low temperature and constant temperature reaction bath, stirring, thermometer, reflux condenser, add 100ml tetrahydrofuran, 27.6g (0.11mol) ethyl N-(4-chlorophenyl)piperazine-1-acetate, 1.8 g sodium ethoxide, control the temperature of the reactor at 0-10°C, stir to obtain N-(4-chlorophenyl)piperazine-1-ethyl acetate tetrahydrofuran mixture, dropwise add 22.8g (0.12mol) of 5- The 5-methoxytryptamine tetrahydrofuran solution composed of methoxytryptamine and 100ml tetrahydrofuran was controlled at a reaction temperature of 5-10°C for 4 hours, then slowly raised to 30°C, and the reaction was continued for 4 hours, and the obtained reaction solution was post-treated.
[0030] Add 5wt% dilute hydrochloric acid to wash until the pH value of the reaction solution is 5.8, wash the reaction solution twice with 20ml and 60ml saturated sodium chloride solution after liquid separation, separate the liquid, dry with anhydrous sodium sulfate, filter, and recover t...
Embodiment 3
[0032] In a reactor with a low temperature and constant temperature reaction bath, stirring, thermometer, and reflux condenser, add 150ml 2-methyltetrahydrofuran, 24.3g (0.10mol) N-(4-fluorophenyl)piperazine-1-acetic acid Ethyl ester, 2.3g sodium ethoxide, control reaction temperature 5-10 ℃, stir to obtain N-(4-fluorophenyl)piperazine-1-ethyl acetate 2-methyltetrahydrofuran mixed solution, drop in 40 minutes by 28.5 5-methoxytryptamine 2-methyltetrahydrofuran solution composed of g (0.15mol) 5-methoxytryptamine and 100ml 2-methyltetrahydrofuran, reacted at the same temperature for 3 hours, then slowly raised the temperature to 50°C, and continued the reaction for 4 Hours, the resulting reaction solution was post-treated.
[0033]Add 10wt% dilute hydrochloric acid to wash until the pH value of the reaction solution is 5.6, wash the reaction solution twice with 20ml and 60ml saturated sodium chloride solution after liquid separation, separate the liquid, dry with anhydrous sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com