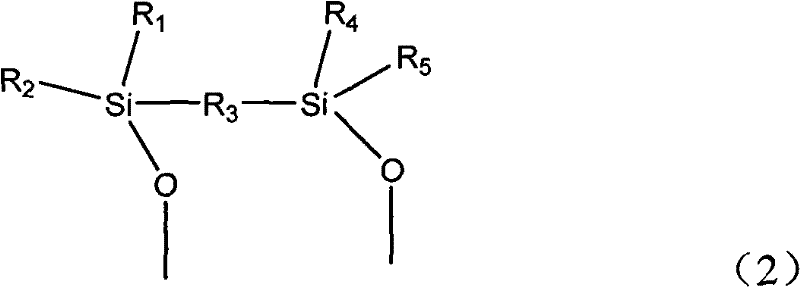
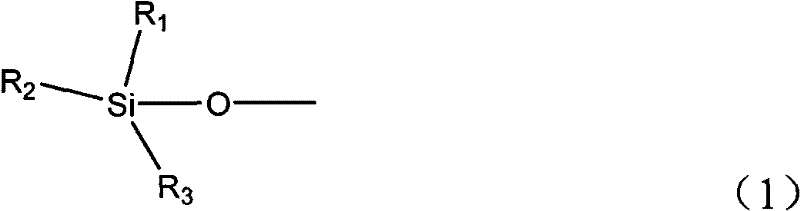Catalytic conversion method of ketone and alcohol
The technology of a catalyst and a hydrogenation catalyst is applied in the field of hydrogenation of ketones to prepare alcohols and dehydrogenation of alcohols to ketones, and achieves the effects of long service life, high selectivity and reduced side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Take 50 grams of clover-shaped Ni-Zn-K / diatomite catalyst with a diameter of 3mm (volume 72ml, the mass percentage content of Ni, Cr, Mo and K are 28%, 12% and 3.5% respectively, and the balance is diatomite ), the catalyst was charged into a fixed-bed reactor (25 mm in diameter, 1000 mm in length, with six temperature display control points). After the reactor temperature was stabilized at 80 °C, hydrogen containing 3 vol% methyltriethoxysilane was introduced into the reactor, the flow rate was controlled at 300 ml / min, maintained at 80 °C for 2 hours, and then heated to 120 °C. After the temperature stabilized The hydrogen gas containing methyltriethoxysilane was stopped for 1 hour, and nitrogen was added to cool down to obtain catalyst Cat-1.
[0059] Comparison of Ni-Zn-K / diatomite and Cat-1, the characteristic peaks of methyl groups on Cat-1 (~2970 cm) by Fourier transform infrared spectroscopy (FTIR) -1 ) was significantly stronger than that of Ni-Zn-K / diatomite,...
Embodiment 2
[0063] Take a strip of Cu-Cr-Ba / Al with a diameter of 3mm 2 O 3 -SiO 2 Catalyst 15g (produced by Beijing Institute of Chemical Industry, volume 24ml, mass percentage content of Cu, Cr and Ba are 28%, 12% and 2% respectively, the balance is Al 2 O 3 -SiO 2 ), put the catalyst into a 500ml there-necked bottle, the three-necked bottle is placed in an oil bath, one of the three-necked bottle is connected to a cooling coil, the other is connected to a thermometer, and the other is connected to a feed port. First, pour 80 ml of p-xylene into the three-necked flask, and after the temperature of the reactor is stabilized at 110° C., pass 8 ml of hexamethylsilazane into the reactor. After maintaining at 110°C for 1 hour, the temperature was raised to 140°C. After the temperature was stabilized, the temperature was maintained for 1 hour and then cooled down. The catalyst was taken out and dried in an oven at 160°C for 3 hours to obtain catalyst Cat-3.
[0064] Comparison of Cu-Cr-B...
Embodiment 3
[0068] The catalysts of Example 1 and Comparative Example 1 were respectively applied to the gas-phase hydrogenation of acetone to isopropanol. The hydrogenation reactor is a 100ml isothermal fixed bed, and the catalyst is 10.0g. The process conditions of the hydrogenation reaction are shown in Table 1 (selectivity refers to isopropanol selectivity). After 800h of reaction, the carbon deposition amount was compared by thermogravimetric-mass spectrometry.
[0069] The reaction conditions are shown in Table 1, wherein the molar ratio of hydrogen to acetone is 8.0. The results are shown in Table 1. The experiment shows that compared with the existing method, the method of the present invention has high target selectivity, low carbon content on the catalyst surface area, and low deactivation rate.
[0070] Table 1 The catalytic reaction performance of the catalysts of Example 1 and Comparative Example 1
[0071]
[0072] Determination of carbon deposition: thermogravimetric-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com